Transforming access to medical implants: How PrinterPrezz and Additive Manufacturing will improve global healthcare
Some companies have bolder missions than others. Whilst Elon Musk leverages metal Additive Manufacturing to transform space exploration, the founders of PrinterPrezz, Alan and Alexis Dang, Kishore Karkera and Shri Shetty, are aiming to do something equally bold with the same technology: bring safe, affordable, right-fit medical implants to the 97% of the world that can't currently access them. Todd Grimm interviewed Alan Dang and Shri Shetty to discover more. [First published in Metal AM Vol. 8 No. 2, Summer 2022 | 20 minute read | View on Issuu | Download PDF]

Casual awareness of PrinterPrezz, which was incorporated in 2018, would lead you to believe that it is just a medical device company that leverages Additive Manufacturing. However, it is much more: it is a unique company on a mission. For one, its Vertex Manufacturing division is AS9100D certified for aerospace and ITAR registered (a regulatory regime to restrict and control the export of defence and military-related technologies), supporting the United States’ aviation and aerospace industry with advanced manufacturing, executing many projects in secrecy. More publicly, one of PrinterPrezz’s primary missions is to change the medical device market as a disruptor, innovator, and market creator.
The company’s stated focus is the production of medical devices and supporting instruments in high volumes with lower costs, all while accelerating the design-to-release timeline. As Shri Shetty, CEO, explains, “We deliver equitable access to life-changing medical devices.” To achieve these lofty goals, the company uses technology, such as metal Additive Manufacturing, process and procedural refinements, and extensive, multi-disciplinary experience. “The value is not only in that we can take devices to market faster. The value is also that we can reduce costs exponentially by using advanced technologies that overlay on our digital backbone.”
Shetty also stated, “The reason we founded this company is that there are a lot of benefits in patient outcomes that have grown from technologies such as Additive Manufacturing and those for semiconductor production.” However, control over medical devices is held by a small number of companies that provide the benefits to a small part of the global population. The vision of the four founders is to change that and disrupt the medical device market.
Combining experience from many industries

PrinterPrezz’s advantages begin with its multi-disciplinary team which brings medical and manufacturing expertise to the business’s operating principles. Initially, this came from the four co-founders, but with strategic additions, the breadth and depth of the company’s knowledge have grown.
The founders include brothers Dr Alan Dang and Dr Alexis Dang, who share the title of Chief Medical Officer; Kishore Karkera, Chief Operations Officer; and Shri Shetty. Their varied backgrounds formed the baseline of the company’s visions, plans and actions.

Alan and Alexis are board-certified, licensed surgeons with academic practices based out of the University of California, San Francisco, and San Francisco Veterans Administration Hospital. Alan is a spine surgeon, and Alexis is an orthopaedic sports surgeon. “We have the mindset of giving patients the best and most cost-effective care,” stated Alan.
As practising surgeons, the Dang brothers have a real-world understanding of the problems that exist in the medical industry and the needs of clinicians, hospitals and patients.
In 2012, Alan and Alexis bought a MakerBot Replicator 2X. At the time, this polymer AM machine was considered experimental, and no one knew how to make it work. Unaware that it was not expected to make parts, good ones or bad, Alan and Alexis got the machine to work through diligence and determination. “We completely rebuilt the nozzles and figured stuff out on our own. That adventure gives us bragging rights,” said Alan. That first desktop Additive Manufacturing experience started these doctors on the path to co-founding PrinterPrezz.
Karkera brings digital experience, having worked for Apple for eleven years. During that time, he spearheaded the production support group in iTunes operations, building its processes from the ground up. He also headed operations groups within Apple Maps and the App Store. He blends technical, operational, and leadership skills.
Shetty is from the semiconductor industry, where he worked for twenty years with companies like Applied Materials and Ultratech. Having run semiconductor and Additive Manufacturing facilities, he has the eye and experience for process innovations that drive efficiency, increase manufacturing yields, and lower costs.
“By combining [these varied experiences], we can take life-changing medical devices to market and reduce cost exponentially, which opens large markets, both in the US and internationally,” explained Shetty. PrinterPrezz used that wealth of experience and know-how to create an end-to-end platform that spans product design to distribution.
As the team has grown — it is now at seventy employees — the depth and breadth of its experience has expanded. For example, nearly a quarter of PrinterPezz’s team are medical doctors, seasoned medical device industry professionals, or graduates of translational medicine or biodesign programmes. Originally focused on in-house Additive Manufacturing, nanotechnology and integrated medical regulatory processes, the acquisition of Vertex Manufacturing has brought broader conventional manufacturing capability to its resource pool.
“We have twenty veterans of the medical device industry,” stated Shetty. “We have doctors that have given up their practices to join PrinterPrezz. We also have people with twenty-five-year careers at companies like Apple and in the semiconductor industry. We round that experience out with team members that have thirty years of experience in CNC machining and Additive Manufacturing.”
In the Additive Manufacturing world, the company’s acquisition of Vertex Manufacturing was big news because it included the addition of Greg Morris, an industry veteran and early metal Additive Manufacturing pioneer, to the team. Morris, who was an early advisor to the company, now serves as PrinterPrezz’s Chief Technical Officer.
Vertically integrated operations
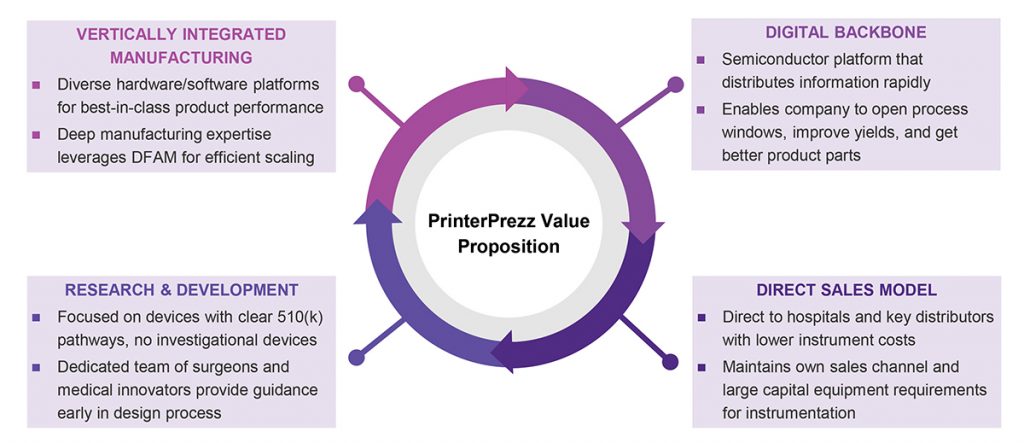
Another vital aspect of PrinterPrezz is its vertical integration, which covers design through distribution (Fig. 4). Typically, medical device manufacturers will turn to their external supply chains for many of the eighteen significant steps in creating, making and delivering implants and instrumentation. More than half of the global market for orthopaedic manufacturing is with contract manufacturers. According to Shetty, nine to twelve suppliers can be in a traditional supply chain, making the process, workflow and information flow challenging. “It’s extremely complex. Each supplier controls its data. It becomes a very complicated supply chain from start to finish,” he said.
As an example, Alan explained, “There are companies that help you prototype, but the problem is that they don’t understand the complete end-to-end process, including FDA reviews.” He also cited design decisions that don’t consider all aspects of the manufacturing process. In terms of elevated implant costs, Shetty noted that each supplier needs to have a reasonable gross margin with the piecemeal approach. When stacked across twelve suppliers, the result is very high medical device costs that are passed on to hospitals and patients.
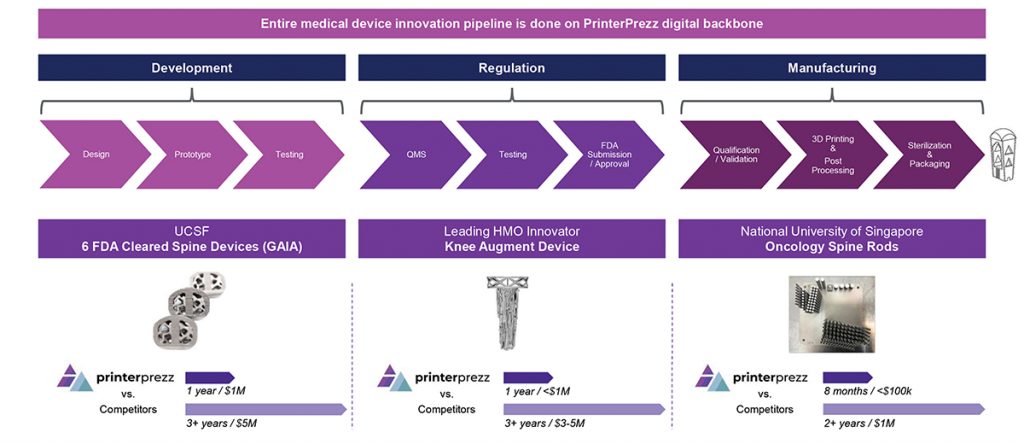
Within PrinterPrezz, parts and data flow seamlessly, and all decisions are made with an eye to the impact on downstream processes. Shetty added, “Everything from design through ready-for-sterilisation is done in-house.” The outcome of this vertical integration is measurable in terms of both cost and time, he explained: “We’ve taken six spinal devices through the FDA in less than a year for less than one million dollars, which historically would have cost tens of millions of dollars and taken three-to-five years” (Fig. 5).
Today, the PrinterPrezz process chain resides within four US facilities and one international facility. Innovative ideas are advanced at the company’s Fremont, California, headquarters (or its new Singapore location), ported to its New Jersey regulatory operations and passed to the Ohio manufacturing facility. A Massachusetts advanced development site focuses on enhancing additively manufactured devices with next-generation nanotechnology surface modulation. A digital backbone connects all of these facilities and their operations.
As Shetty explained, “Our value is not only that we can make things faster. The added value is that we can reduce costs exponentially using advanced technologies that overlay on our digital backbone. We have built a semiconductor-like and Apple-like backbone that lets us get yields in the high 90s [as a percentage]. In the traditional manufacturing process, the yields are in the 60s.” Combined with vertical integration, the digital backbone facilitates sound decisions across the entire process chain. Shetty added, “If a company is only worried about a single step in the process, it is not going to achieve high yields.” Considering that all of its medical devices are produced with Additive Manufacturing, yields above 90% are even more impressive. The digital backbone will also facilitate future expansions by PrinterPrezz, both within the United States and internationally.
Clinical innovation
According to Alan, “The innovation piece is an essential part of the company.” He cites actions like leveraging technology used to make better computer chips and better software that Shetty and Karkera brought into the company. Yet, as he quickly pointed out, “The innovation on the clinical side is very important. If you look at our implants, they’re designed to be transparent to the patients and the surgeons so that they are not required to relearn an entirely new technique.” So, PrinterPrezz’s innovations align with making implants better, solving problems and addressing underserved markets.
As with other aspects of the business, the company looks outward for innovative ideas through partnerships and collaborations from within healthcare networks. For example, PrinterPrezz has an IP agreement with the Regents of the University of California, which is the governing board, allowing the company to bring new ideas from other clinicians to market. Alan stated, “These ideas are normally too early for big companies to develop or too complex for someone to do it in their garage or research lab. They just don’t happen.” PrinterPrezz, on the other hand, has unique expertise supported by a business model that facilitates bringing these innovations to market collaboratively with the university.
“It is about laying the groundwork rules for IP so that innovative surgeons and clinicians don’t have to worry about all the aspects that go into bringing a new medical device to market and universities retain their fair share of the IP,” he said. Considering the substantial barriers to entry, the solutions to problems experienced in the operating room or clinic would stall at the idea stage if a company like PrinterPrezz lacked the capabilities, talents and appetite for bringing new, innovative products to market while working within the university technology transfer system.
Shetty added, “The main motivation is the fact that the value of the technology isn’t in the yield or capabilities. The value of the technology is the products that are created.” Alan agreed, “We don’t want just to make things cheaper – we want to make better things even cheaper.” For an example, he cited the innovative work of the Massachusetts nanotechnology lab to address issues around healing and surgical site infections, problems that affect all surgeons and all patients.

The nanotechnology, which will be applied to additively manufactured devices, alters surface energy, which affects the way the implant behaves biologically. Having filed patents and performed independent lab testing, PrinterPrezz is poised to improve healing and bone growth through two semiconductor processes: nano etching and atomic layer deposition (coatings at nanoscale). Combined with latticed designs and the consideration of design and part orientation impact on surface quality, the company will deliver better outcomes for those that are surgical candidates and open the door to those that might otherwise be denied due to preexisting illness or comorbidities.
“Medical device innovation has traditionally focused on improving mechanical performance and improving surgeon or patient ergonomics,” Alan said. “Nanotechnology adds the fundamental concept of helping patients heal after the surgery is done, without interrupting the clinical workflow.”
What is PrinterPrezz doing now?
“New medical technologies are often introduced as high-end devices for the 3% of the world that already has access to advanced healthcare solutions. We are instead using our technology advantages to better serve the 97% that are underserved or ignored,” Alan explained. “Because of our cost advantages, we can make a reasonable profit while impacting a lot of lives in areas that cannot afford current solutions.”
PrinterPrezz is not going after custom, patient-specific medical devices. Instead, it focuses on high-volume production with a high product mix. Essentially, its definition of mass customisation is to build a multitude of variations of each medical device tailored for shape, size, and topology. Except for commodity items like screws and plates, everything that PrinterPrezz produces is made with Additive Manufacturing. The inherent batch-to-batch flexibility in the product mix is a critical piece of the company’s plan to deliver a significant number of product variations.

The limited impact potential of customised, patient-specific devices, based on the percentage of the population that can afford them, is a primary reason for PrinterPrezz to address the high-volume, high-mix opportunities. While some procedures require custom solutions, most are well-served by standardised devices that come in a wide range of sizes. A second reason is that processes that support patient-specific devices, in the areas of regulatory control and CT scanning to additively manufacturable models, are not as evolved as those for Additive Manufacturing and the materials and software that support it. Shetty said, “We see the pathway to doing these custom devices being limited by the demand and the regulatory agencies in today’s environment. Maybe there will be a valid customisation market in the future, but it will never be as big as the mass-customisation market.”
Presently, PrinterPrezz has six FDA-cleared spinal implants used in the lower back, a seventh FDA-cleared implant for the neck, and an additional nineteen devices in the pipeline. The company is also interested in expanding its added-value solutions to address surgical procedures for trauma and extremities.

Shetty stated, “3D printing is one piece of [our solution] that offers the customisation, and semiconductor is the piece that yields attractive costs. But both of them together, with the right people, give you products that can have real impact.” Overlaying advanced digital technology and advanced manufacturing technology enables PrinterPrezz to be cost disruptive in the medical device market.
Solutions for 6.7 billion people
“We see huge markets in the US and Asia that are very cost-competitive, and therefore, not targeted by those using advanced technologies. With our cost structure, we can be a disruptor,” said Shetty. In Asia, he noted, 90% of the market does not have access to reasonably priced, FDA-approved devices, so they turn to knockoffs for an affordable alternative. Alan noted, “Today, we can deliver ‘Made in the USA,’ FDA-cleared devices at a lower cost than anything on the market globally. And we believe we can continue to drive that cost down.”
Another consideration is that medical device companies have historically focused on serving the North American and Western European market, so the product designs are optimised for the typical North American bone structure. That means that the devices may not optimally fit the rest of the world. PrinterPrezz can design and manufacture for different global ethnicities by leveraging its high-volume, high-mix production capabilities.

Alan likened the high-mix capabilities to off-the-shelf shirts with various fits, sleeve lengths and collar sizes. “We can have a core design supported by a size matrix of hundreds of variables. These won’t be manufactured on demand; rather, we will produce them in right-sized batches based on the demand from each region of the world.” This global vision is the reason for the recently opened innovation centre in Singapore. Like the Fremont location, this facility collaborates with regional health clusters to bring solutions to market that address the problems faced by the medical industry and deliver medical devices that address the needs of patients and clinicians.
The use of Additive Manufacturing and semiconductor materials makes the global solution possible. But what makes it a reality is the company’s digital backbone. “We can scale up globally. We aren’t limited by physical assets and disjointed data flows. As we expanded into Southeast Asia, and as we expand into the Middle East or South America, we will be using the same digital backbone. In this way, we become more like Amazon or Uber and much less like a traditional contract manufacturer,” said Shetty. As it expands, PrinterPrezz will add innovation centres, micro-factories and regulatory groups that can support the regional needs of countries beyond the US and Singapore.
Shetty stated, “PrinterPrezz is in a heavy expansion mode, and we are getting close to securing Series B funding to fuel that growth.” However, he added, “We focus on the implants; we focus on the digital manufacturing; we focus on the globalisation. We focus on the devices and the customer.” With operational structures, business processes and advanced technologies that support growth, the company can scale.

Shetty continued, “As we scale, we can cut our costs even further.” For evidence, Shetty and Alan reference the flat-screen TV market. At scale and with efficient processes, what once cost $10,000 is now offered for $1,000 with the same or better profit margins. Shetty explained, “By combining everything we have assembled, PrinterPrezz can take life changing medical devices to market while reducing cost exponentially… that opens large markets, both in the US and internationally.”
Conclusion
The PrinterPrezz story is impressive in many ways, but, in our conversations, it was shared with humility and without hyperbole or conjecture. As Alan said, “We underpromise and over deliver. Our focus today is delivering state-of-the-art medical implants at an affordable cost, leveraging already proven methods from semiconductor manufacturing and e-commerce. Our nanotechnology programme is an investment for the future of hypermaterials, is built upon decades of science related to surface energy, and has already been evaluated by an independent third party lab in a large animal study. Lastly, our aerospace additive expertise and operational confidentiality is likely among the Top 3 in the industry. We have seen tremendous growth this year and additively manufacture for some of the biggest household names in that industry.”
This is a company to watch as it innovates, disrupts and opens new markets for medical devices.
Author
Todd Grimm
Todd is a thirty-two-year veteran in Additive Manufacturing and president of T. A. Grimm & Associates. He is a consultant, presenter, writer and author.
Contact
Scott Zafiropoulo
SVP, Marketing
PrinterPrezz
47929 Fremont Blvd.
Fremont, CA 94538
USA
Locations and areas of expertise

PrinterPrezz headquarters Fremont, California
This location is an innovation centre where ideas are born, devices are created, new product introductions (NPI) are conducted and technology readiness (T.R. 1 through to 4) is achieved. It is home to the company’s doctors and where new material process, for example PAEK, development occurs.
Fremont works with large hospital networks such as UCSF (University of California San Francisco), Stanford, and medical innovators like CZ BioHub to collaboratively bring device concepts to market.
Addivation Medical Chester, New Jersey
Staffed with former Zimmer, Stryker, and Biomed employees, this location serves the ‘medical device technologist’ role of taking new concepts through the FDA regulatory process.
Vertex Manufacturing Cincinnati, Ohio
When PrinterPrezz was founded, this independent company was its exclusive post-processing partner. Today, it is the company’s manufacturing arm, offering additive and traditional manufacturing. Beyond PrinterPrezz products, Vertex also serves as an AS9100D certified, ITAR registered contract manufacturer for non-medical products in industries such as aerospace and defence.
Upon completing the coming facility expansion, Vertex will become the first digital robotic facility in the US for medical and aerospace Additive Manufacturing production.
PrinterPrezz is technology and vendor agnostic so that it can pair the best solution with each application. Its stable of metal Additive Manufacturing technologies includes metal AM machines from Arcam (GE) and Concept Laser (GE), 3D Systems, EOS, Desktop Metal, Velo3D and Xerox. The company also has several high-temperature material polymer printers.
PrinterPrezz – Nanotech Lab Boston, Massachusetts
After manufacturing, this advanced development facility applies nanocoatings or nano-etches (from the semiconductor world) to improve material performance in areas such as infection mitigation and bone growth.
PrinterPrezz SG Singapore
Established before the global pandemic to support research and development projects coming from the national health clusters of Singapore, PrinterPrezz Singapore will be launching a full innovation centre this autumn to perform many of the same medical device development functions as the Fremont location, focusing specifically on the needs of the region.
Why the name PrinterPrezz?
The company had a vision from its onset to break down barriers to medical device access. That vision led to the company’s name. It saw itself in a context aligned with Gutenberg and the advent of the movable-type printing press – hence PrinterPrezz.
Before Gutenberg, books were studied by the elite that could afford them, making knowledge a privilege that few could access. With the printing press, books were produced in greater numbers at far less expense and, as a result, the spread of knowledge, discoveries, and literacy swelled.
With the intent to offer lower cost solutions for medical devices produced in high volumes using a digital workflow, the company adopted the PrinterPrezz name.







