Titanium powder pyrophoricity, passivation and handling for safe production and processing
As Additive Manufacturing moves out of the prototyping space and into production facilities with multiple machines, the importance of handling and processing powders, particularly titanium, becomes ever more relevant. In this article Dr Andrew Heidloff and Dr Joel Rieken, from Praxair Surface Technologies, Inc., review best practice when handling and storing titanium powders for AM. Titanium powder can be safely produced, processed, stored and shipped using appropriate precautions, however under certain conditions it can become quite hazardous. These hazards can be mitigated by following the suggested precautions reviewed below. [First published in Metal AM Vol. 2 No. 1, Spring 2016 | 15 minute read | View on Issuu | Download PDF]

As companies enter the Additive Manufacturing sector, they are often for the first time introduced to the world of Powder Metallurgy. The following descriptions and recommendations are meant to acquaint those unfamiliar with Powder Metallurgy with the correct resources. For those that are new to this segment and are currently using or producing powder, they are encouraged to test the powder with a trusted and certified third party for pyrophoricity and explosibility characteristics, as this will form the foundation of many choices that will be made in order to operate a facility under safe conditions.
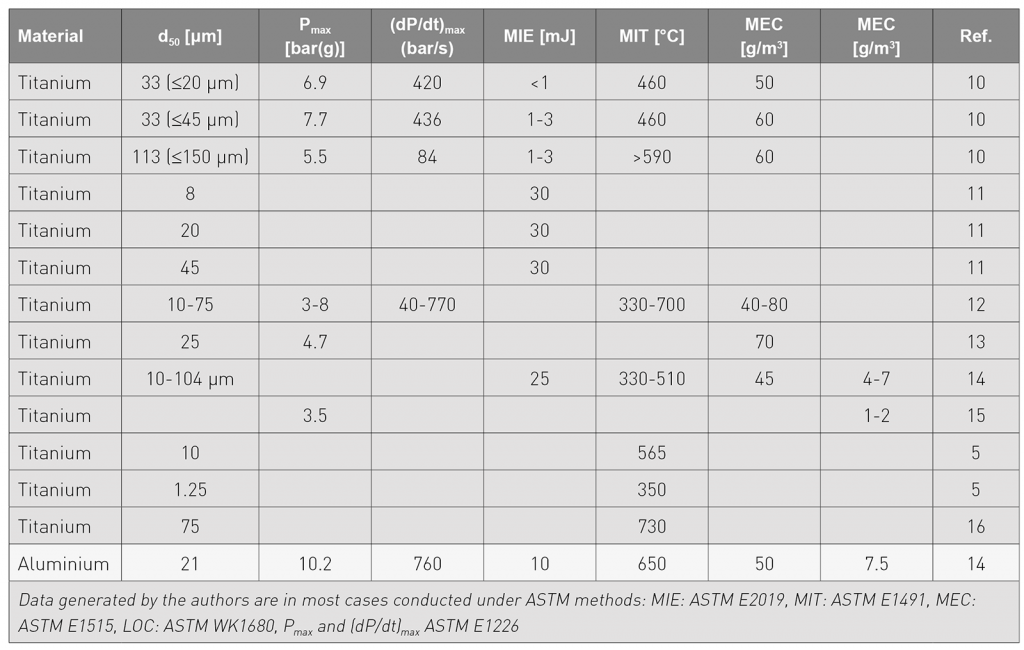
This testing approach consists of a screening test to determine if the powders are capable of initiating and sustaining an explosion (20 L chamber per ASTM E-1226), followed by: Minimum Ignition Energy (MIE), Minimum Ignition Temperature (MIT), Pmax, Kst, Limiting Oxygen Concentration (LOC) and Minimum Explosible Concentration (MEC). All of these characteristics will aid in understanding what is important to safety in a given facility and scenario.
Why is titanium reactive?
Titanium is a well-known material to be characterised as flammable under certain morphologies. Titanium and its alloys have a great affinity for oxygen and will form a native 2-7 nm TiO2 layer instantly if a clean metallic surface is exposed to air at room temperature. This film prevents further oxidation from taking place and protects the underlying metal powder. When heat is applied, either through a thermal source or a spark, the powder can generally or locally heat to the point of thermal runaway or burning. The consensus mechanism of self-sustaining thermal runaway of titanium powder occurs by means of ion diffusion through this native TiO2 film on the titanium powder [1, 2]. As the micron size of the powder decreases, the specific surface area (in units of m2/g) increases at a rate of 6/d where d=particle diameter. In context, to fill a typical Additive Manufacturing machine with 45 kg of titanium powder, with an average particle size of 20 µm, this powder will have enough surface area to cover over 3000 m2. Generally, titanium powders with a particle size < 45 µm are considered a flammability hazard.
When describing a reaction of any metal powder, there are three categories into which each reaction may fall: 1) stagnant, 2) freely aspirated and 3) conveyed. Stagnant powder reactions generally are a result of powder that collects on a horizontal surface and ignition is typically from a heat source, as a more significant source is necessary to ignite a stagnant bed of powder. When powder is dispersed in the air, the fine powders may stay aloft creating a cloud. Aspiration of powder, and specifically titanium powder, does not automatically mean the cloud will ignite spontaneously. However, if the temperature threshold or spark energy necessary for ignition is met, rapid oxidation of powder can occur as it mixes with oxygen from the air. This is a result of no thermal heat sink of other powders or materials in near proximity to the powder cloud allowing it to reach a much higher temperature and propagate to other powders, which may result in a large pressure increase and possible explosion.
Ignition can come from a variety of sources, which will be discussed throughout this article. Thermal exposure to temperatures of 300-700°C can cause ignition of titanium powder despite the native oxide layer (i.e., minimum ignition temperature or MIT). Spark ignition can come from a variety of sources including static electricity build-up, electric components and friction/impact of metal components. Titanium powder can have minimum (spark) ignition energies (MIE) of 3-30 mJ.
Powder production
After atomisation, powders are traditionally collected in a cyclone system. These powders are typically non-passivated. The transfer of these non-passivated powders from the atomisation cyclone to ancillary process containers is considered to present a high risk of thermal runaway, which may require breaking of the inert gas seal and exposure to oxygen with high potential for powder aspiration. To overcome this problem, non-passivated powder requires exposure to air (or a reactive gas) to passivate at room temperature, a very time consuming and potentially dangerous process. As an example, passivation of 215 kg of aluminium powder was conducted in a powder collection canister after atomisation, requiring a 20 hour cool down (below MIT), followed by a 1.5 hour passivation period [3]. While canisters can be isolated and moved for passivation, this process concentrates a large quantity of nascent surface powders (i.e. highly reactive) in a confined vessel, which is not ideal.
A novel passivation approach
As a solution to this problem, Praxair Surface Technologies, Inc. uses a novel in-situ passivation process that prevents further oxidation of the powder during exposure to air, thus minimising any exothermic reaction, thereby greatly diminishing the possibility of thermal runaway or burning of powder. Using Praxair’s in-situ process, titanium powders are passivated prior to reaching the cyclone collection and are deemed safe to handle after dropping below the aforementioned MIT (300-700°C in air). This not only increases the productivity of titanium powder production, but also greatly diminishes the hazards of the powder.
The ability to add a specific passivation layer to the titanium powder without greatly affecting the powder making process requires the formation of an oxide shell in-situ after the powders initially solidify and descend downwards within the atomisation chamber. The most important aspect of in-situ passivation is the generation of a layer similar (in thickness and chemistry) to the native oxide film that will form on the surface of titanium at room temperature (i.e., a 2-7 nm thick oxide) [4-7]. Oxide thickness becomes extremely important because of the extremely large surface area described above. Ideally, the total oxygen content should stay below 1300 ppmw (0.13 wt.%) for a 20 µm particle, which requires a target titanium oxide thickness of ~2-3 nm if the bulk material contains less than 1000 ppmw of O2. If the target oxide shell thickness of 1-3 nm can be produced then no additional oxidation should take place when exposed to air at room temperature for extended periods of time.
The production of a 1-3 nm oxide shell in-situ on atomised powders is accomplished by employing both thermal and physical energy balance models to accurately predict the temperature vs. distance profile of the atomised powders (as they proceed through the spray chamber). Passivation halos are used to inject a specific reaction gas (for example an Ar-O2 mixture) at a predetermined vertical position within the atomiser in order to achieve ideal oxidation kinetics.
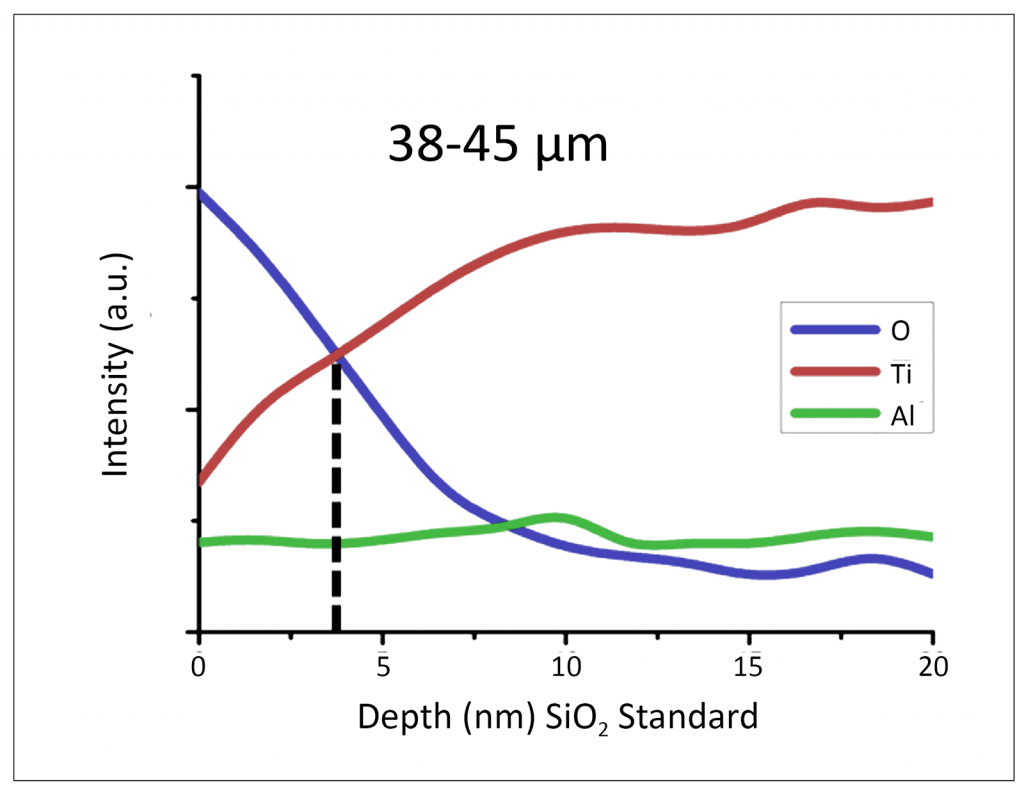
Fig. 3 shows an Auger Electron Spectroscopy depth profile of the in-situ passivated Ti-48Al-2Cr-2Nb powder (dia. 38-45 µm) shown in Fig. 4. The crossover point is the general consensus of an oxide thickness and is highlighted at ~3 nm. This analytical tool provides an example of the novel passivation process.

Post-production processing and handling safety
The post-processing of titanium powder undoubtedly will utilise electrical equipment from sieves, blenders, feeders, etc. This challenge also presents itself to users of Additive Manufacturing equipment. When considering electrical installations involving any flammable substance, it is highly recommended to reference National Electrical Code, NFPA 70, particularly articles 500 to 504. These sections describe the recommended best installation practices for electrical equipment in the presence of a hazardous material. Class II is relevant to combustible dusts (e.g., metal powders) and, within Class II locations, there are Divisions I and II. To determine which division a process/material may fall into, the reader is directed to read these descriptions carefully.
Beyond electrical installations, certain procedures/guidelines should be considered when handling titanium powder.
Guideline I: Avoid any condition that will suspend or float powder particles in the air, creating a dust cloud
1) Minimise accumulation of dust on floors, walls and other surfaces by complying with good housekeeping practices in all areas where titanium powder is stored or handled.
2) In transferring titanium powder, dust clouds should be kept at an absolute minimum. Handling should be slow and deliberate. Both containers should be bonded together and should utilise a grounding strap.
3) All powder transfers and spills should use grounded, conductive and non-sparking tools; while synthetic bristle brushes and plastic should be avoided.
Guideline II: Eliminate all sources of ignition in powder handling areas
1) Avoid MIT personnel sources by not allowing open lights, smoking, lighters or matches in areas where titanium powder is present.
2) To prevent MIT sources of a processing nature, items such as blow torches, welding torches or other open flames are prohibited. No flame, spark-producing or propellant-actuated tools or activities should be carried out in an area where titanium powder is present. Where activities such as welding, cutting, grinding or use of portable electric tools is necessary, a “hot work” permit system is strongly recommended.
3) Avoid MIT sources by minimising friction sparks by avoiding metal-to-metal or metal-to-concrete contact of tools as these impacts can produce sparks that will ignite titanium powder. Non-sparking tools must be used where there is a possibility of impact sparks.
Guideline III: Eliminate the generation of static electricity, where possible, and prevent static charge accumulations
1) Bonding and grounding of machinery to remove static electricity produced in powder operations are vital for safety. Bonding and grounding should be done in accordance with the latest versions of Recommended Practice on Static Electricity, NFPA 77, and Standard for Combustible Metals, NFPA 484.
2) All moveable equipment, such as bins, containers and scoops, should be bonded and grounded during powder transfer by the use of clips and flexible ground leads. Ground-conducting floors and/or mats in conjunction with static dissipative footwear/casters are an attractive solution for personnel and movable equipment. Care should be taken to ensure the proper resistance to ground for such designs.
3) During transfer, powder should not be poured or slid on nonconductive surfaces as this can lead to static electricity buildup. A static-dissipative or conductive liner is also recommended.
Guideline IV: Take steps to limit the size of a fire or explosion and to hold any resulting damage to the very minimum
1) The storage area for titanium powder is recommended to be separate from handling of powder. Rooms of noncombustible or limited-combustible construction are encouraged. Titanium powder should not be stored in areas containing incompatible materials such as flammable liquids, oxidisers, organics, fuels or other combustible materials due to the differences in firefighting methods. Storage methods should also be considered by stacking containers properly with ample aisle space and keeping stack height to a minimum.
2) Keep all titanium powder storage containers tightly sealed to prevent accidental dust generation and to prevent possible contamination from moisture or other dusts.
3) All containers in work areas should be closed and sealed. Only those in use for removal of material should be open at any time and should be closed and resealed as quickly as possible. This not only assures greater safety against fire from external sources, but also prevents possible entrance of tramp contamination material or water from the air.
4) Consider the use of an inert cover gas, which can replace air, as this can be valuable in minimising the hazards in many operations, particularly where it may be impossible to ensure that all sources of ignition are eliminated. Utilisation of an inert cover gas will be linked to the LOC determined during powder explosivity characterisation.
Regulation of dust
While practices are employed to prevent clouds during titanium handling, such as restricting compressed air or any other controls that are put in place, undoubtedly a certain amount of dust will be generated within a facility. Regulation of dust is necessary and, while dust control could be considered to fall into any one of the aforementioned guidelines, it is laid out separately here, as dust control is of primary importance.
Dust is one of the most likely sources when a safety incident occurs in a titanium powder facility. Standard industrial central vacuum systems/cleaners should not be used for cleaning as accumulation of dust on a dry filter can create a safety hazard. Dust ventilation systems should be rated for the proper NFPA environment class. Special explosion prevention systems, specifically approved for use with combustible metal dusts, are highly recommended. These systems maintain necessary ventilation air velocity to prevent powder settling in duct work, as well as utilising duct work that is Pmax and Kst rated in the event of an explosion within the duct. These design considerations are another reason for combustibility and explosibility powder characterisation.
The role of personnel
Use of personal protective equipment (PPE) is vital for safely working with titanium powder. Personnel should ground themselves using a wrist strap or grounding plate with conductive shoes at workstations. When static-dissipative footwear and mats and/or flooring are used, testing preventative maintenance should be employed to ensure that the static dissipative properties have not deteriorated. Trousers and shirts should not have cuffs, where dust might accumulate, and should be made of closely-woven fire resistant/fire retardant fabrics, which tend not to accumulate static electrical charges. Respirators are highly recommended as inhalation of powders can easily happen, particularly if the proper dust regulation equipment is not installed. Full face or half face respirator choices should be made based on the personnel tasks and responsibilities. PPE should also include safety glasses, leather gloves and, potentially, face shields.
Safety must be a critical part of the facility culture. Continual efforts should be taken to educate personnel and improve equipment safety. If a highly safety-conscious workforce is combined with the execution of procedures, methods and equipment, such as those discussed here, titanium powder can be produced and/or used safely.
Authors
Dr Andrew Heidloff, Dr Joel Rieken
Praxair Surface Technologies
1500 Polco Street
Indianapolis
Indiana 46222
USA
Tel: +1 317 240 2500
Fax: +1 317 240 2380
Email: [email protected]
www.praxairsurfacetechnologies.com
References
[1] K.L. Erickson, Jr. J.W. Rogers, and S.J. Ward, “Titanium oxidation kinetics and the mechanism for thermal ignition of titanium-based pyrotechnics,” in Internal Pyrotechnics Seminar, 1986, pp. 679-697.
[2] E.A. Gulbransen and K.F. Andrew, “Kinetics of the Reactions of Titanium with O2, N2, and H2,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 185, pp. 741-748, 1949.
[3] G.T. Campbell, R.W. Christensen, and D.L. Oberholtzer, “Low temperature passivated aluminum powder, properties and benefits,” in Advances in Powder Metallurgy and Particulate Processing, 1997, pp. 3-10.
[4] G. Hass, “Preparation, structure, and applications of thin films of silicon monoxide and titanium dioxide,” Journal of the American Ceramics Society, vol. 33, pp. 353-360, 1950.
[5] J.P. Evans, W. Borland, and P.G. Mardon, “Pyrophoricity of Fine Metal Powders,” Powder Metallurgy, pp. 17-21, 1976.
[6] E. Baril, “Titanium and titanium alloy Powder Injection Moulding: Matching application requirements,” Powder Injection Moulding International, vol. 4, pp. 22-32, 2010.
[7] Y. Oshida, Bioscience and Bioengineering of Titanium Materials: Elsevier, 2006.
[8] “Powder handling guidelines for working with titanium powder,” Cristal Global, 2013.
[9] “Recommendations for storage and handling of aluminum powders and paste,” The Aluminum Association, 2006.
[10] Simon P. Boilard, Paul R. Amyotte, Faisal I. Khan, Ashok G. Dastidar, and Rolf K. Eckhoff, “Explosibility of micron- and nano-size titanium powders,” Journal of Loss Prevention in the Process Industries.
[11] Hong-Chun Wu, Ri-Cheng Chang, and Hsiao-Chi Hsiao, “Research of minimum ignition energy for nano Titanium powder and nano Iron powder,” Journal of Loss Prevention in the Process Industries, vol. 22, pp. 21-24, 2009.
[12] V.V. Nedin, “Pyrophoric capability and explosiveness of titanium powders,” pp. 57-66, 1971.
[13] K.L. Cashdollar and I.A. Zlochower, “Explosion temperatures and pressures of metals and other elemental dust clouds,” Journal of Loss Prevention in the Process Industries, vol. 20, pp. 337-348, 2007.
[14] M. Jacobson, A.R. Cooper, and J. Nagy, “Explosibility of Metal Powders, Report 6516,” U. S. B. o. Mines, Ed.: Dept. of Interior, 1964.
[15] A.G. Alekseev and E.S. Kostina, “Determination of dangerously explosive oxygen content in a protective atmosphere for dispersed titanium powders,” in Preduprezhdenie Vnezapnykh Vzryvov Gazodispersnykh Sist., V. V. Nedin, Ed. Kiev: Kaukova Dumka, 1971, pp. 78-85.
[16] E. Beloni and E. Dreizin, “Ignition of Titanium Powder Layers by Electrostatic Discharge,” Combustion Science and Technology, vol. 183, pp. 823-845, 2011.







