Additive Manufacturing at World PM2016: Opportunities for the use of water atomised metal powders
At the World PM2016 Congress, held in Hamburg, Germany, 9-13 October, 2016, two papers discussed the potential for the replacement of gas-atomised powders with water-atomised powders as the raw material for Selective Laser Melting of different alloy types. Dr David Whittaker reports on the work undertaken to assess the viability of the water atomisation process for these materials which could, in turn, offer significant cost savings. [First published in Metal AM Vol. 3 No. 1, Spring 2017 | 15 minute read | View on Issuu | Download PDF]
Microstructures and mechanical properties of Selective Laser-Melted metals with different processed powder
The first of these papers, from Naoyuki Nomura, Keiko Kikuchi and Akira Kawasaki (Tohoku University, Japan), considered the processing of Co-Cr-Mo alloys. Co-Cr-Mo alloys have been widely used as metallic biomaterials in applications such as bone fixation devices, artificial knee joints and dental appliances, because of their excellent strength and good corrosion resistance. Such alloys have been used in the as-cast state with slow cooling and, therefore, show limited ductility because of the precipitation of carbides in the inter-dendritic regions. Also, when carbon is not added to the alloy, a martensitic transformation from the γ phase (f.c.c) to the ε phase (h.c.p) occurs during cooling after casting. The resultant ε phase restricts further deformation. To improve ductility, the Tohoku University group has developed a nitrogen-containing Co-Cr-Mo alloy, where nitrogen acts as a γ phase stabiliser. In alloys with added nitrogen, elongation was dramatically improved when nitrogen and chromium contents were increased in tandem. The group has also reported that the mechanical properties of a Co-29Cr-6Mo alloy were improved by applying a Powder Bed Fusion (PBF) process using a laser compared to a conventional dental casting. The γ phase was predominant in the SLM-built microstructure.
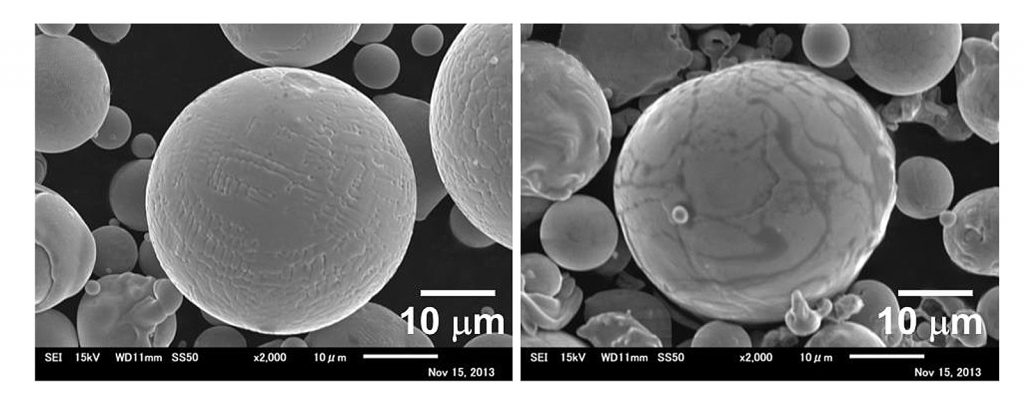
In the present reported study, the focus was mainly on the difference in responses of gas and water atomised powder variants. Co-33Cr-5Mo-0.4N (mass%) alloy powders were prepared by gas and water atomisation. A spherical particle shape with a smooth surface and dendritic traces was observed in the gas atomised powder, whereas an ellipsoidal particle shape with a dark contrast area was seen in the water atomised powder (Fig. 1). The median diameters (d50) of the gas and water atomised powders were 27.5 µm and 24.5 µm, respectively.
Elemental mapping of the water atomised powder identified lower levels of Co and Mo at the area with dark contrast on the surface. However, Si and O were enriched at the surface. Si is generally added to Co-Cr-Mo alloys to improve metal flow in casting and was contained in both of the powders. This observation suggests that Si oxides may have formed during the water atomisation process.
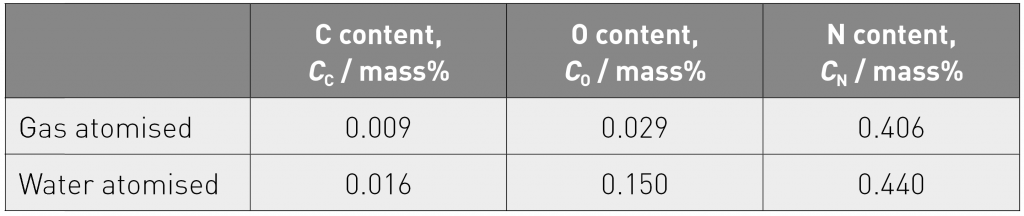
Table 1 shows the C, O and N contents in the gas and water atomised powders. The C and N contents were at very similar values in the two powders, but the O content in the water-atomised powder was about three times higher than that in the gas atomised powder. X-ray diffraction (XRD) profiles for the two powders both showed γ phase peaks, but an absence of ε phase peaks, confirming that the nitrogen addition had achieved its intended objective in suppressing the martensitic transformation. The calculated lattice parameters were very similar for the two powders at 0.3587 nm and 0.3591 nm for the gas and water atomised powders, respectively. Taking this observation together with the measured higher O content in the water atomised powder (reported in Table 1), the conclusion was drawn that the oxygen must have been localised at the powder particle surface as oxides and was not in solution in the alloy.
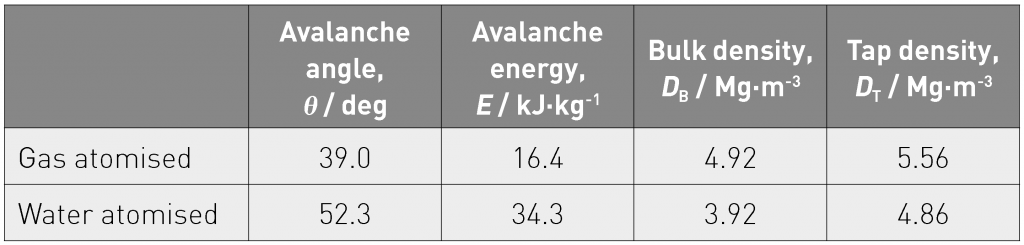
Flowability of the powders was evaluated using a Revolution Powder Analyser from the viewpoint of avalanche angle and energy. These flow characteristics, together with bulk and tap densities, are shown in Table 2.
PBF was performed with both powders using an EOSINT M250 extended machine with a nitrogen atmosphere. The laser power was selected at 150 W or 200 W. Hatching spacing varied from 0.1 mm to 0.3 mm and the laser scan speed and layering thickness were 50 mm/ sec and 0.050 mm, respectively. Using these parameters, dumbbell specimens (3 mm in diameter and 18 mm in gauge length) were prepared for tensile tests.
Fig. 2 shows optical micrographs of the Co-33Cr-5Mo-0.4N alloy built by PBF, with the gas- and water-atomised powders, at a range of different energy density levels. Porosity was also indicated in these micrographs. In the samples built by PBF, porosity increased with increasing hatching distance (i.e. decreasing energy density). It should be noted that the porosity of the PBF built sample with the gas atomised powder was higher than that with the water atomised powder. This difference was more apparent at lower energy density.
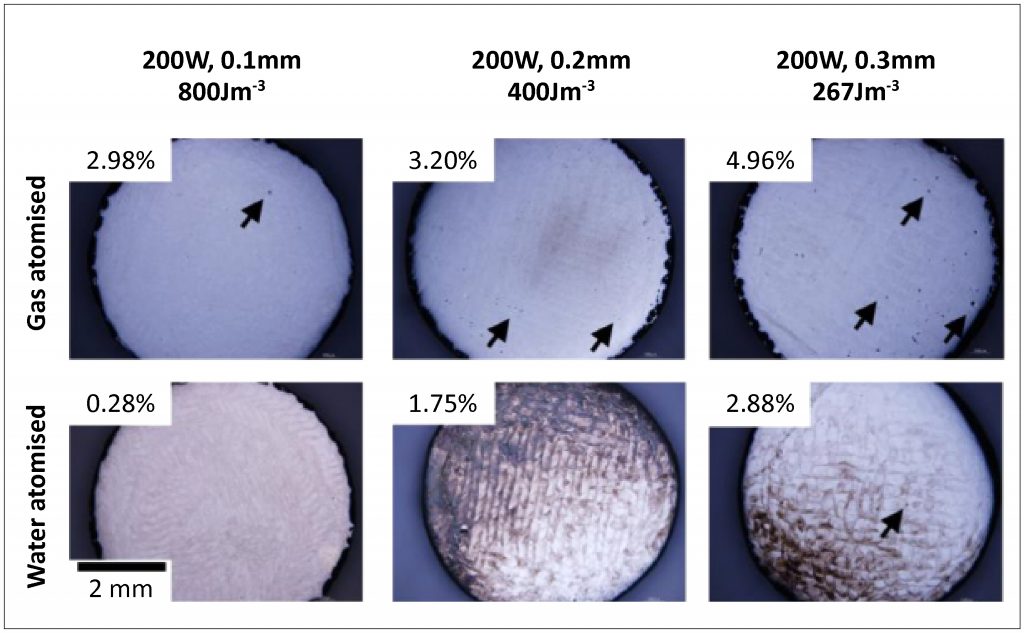
Fig. 3 shows the mechanical properties of test-pieces built by PBF. The mechanical properties of the builds fabricated with the two powder variants show very similar values at higher energy density. However, with decreasing energy density, the builds with the water atomised powder showed superior mechanical properties, compared with those with the gas atomised powders. The authors have concluded that this difference can be related to the respective porosity levels.
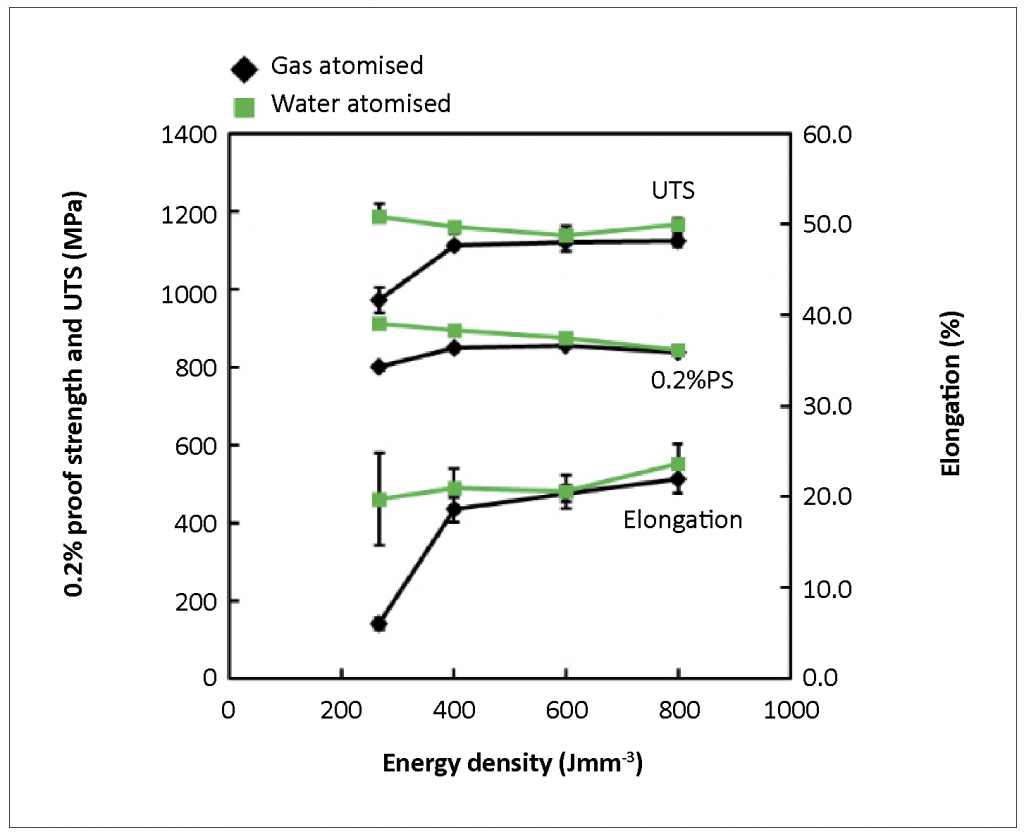
The porosity differences in the PBF builds can be explained with reference to the data in Table 2. The avalanche angle and energy of the water atomised powder were higher than those of the gas atomised powder. The bulk and tap density of water atomised powder were lower than those of gas atomised powders. These data indicate that the flowability of the water atomised powder was worse than that of gas atomised powder. The lower bulk and tap density means higher porosity in the powder stacked layer and this influences thermal conductivity. Normally, thermal conductivity in a material containing higher porosity is lower than that in one containing lower porosity. When the laser was scanned over these two powders at the same energy level, the temperature rise in the higher porosity layer should be higher than that in the lower porosity layer. In addition, thermal conductivity of SiO2 is lower than that of the Co-Cr-Mo alloy and therefore the heat transfer in the water-atomised layer may be hindered by the existence of the Si oxide surface layer. Accordingly, the molten pool with the gas atomised powder could be smaller than that with the water atomised powder. Further investigation is underway to test this hypothesis
Overall, the authors have concluded that the Co-Cr-Mo-N alloy could be successfully built by PBF with either of the two powder variants and that, therefore, the water atomised grade could be regarded as a viable alternative to the gas atomised grade.
Additive Manufacturing using water atomised steel powders
The second paper, from Simon Hoeges (GKN Sinter Metals, Germany), Alex Zwiren and Christopher Schade (GKN Hoeganaes, USA), switched the attention to 316L stainless steel powders. The authors stated that at the current status of the technology’s development, confidence in the integrity of parts manufactured by AM is based on powder characteristics determined largely by the system manufacturers themselves. Two most prominent characteristics that dictate high density part fabrication have been defined as being particle size and sphericity.
To achieve these powder characteristics, gas atomisation has emerged as the most widely used method for AM powder production. However, the very strict specifications imposed on powder size, morphology and chemical composition impact powder yield levels and lead to high prices for acceptable powders for AM.
In water atomisation, jetting pressures can be varied in the range between 2000 and 10,000 psi to obtain desired size ranges and degrees of sphericity. This capability removes the assumed limitations of water atomised powder, in the context of AM, and reduces the differences between water and gas atomised powder to chemical composition differences, including oxygen levels.
In water atomisation, little refining can occur and certain elements cannot be removed. This means that the carbon in the raw material remains in the final product, facilitating the necessity for low carbon feedstock. Likewise, manganese is not removed in the process, meaning that the raw material must also meet maximum requirements for acceptable content. Controlling the manganese in the raw material is important in reducing the oxidation effects that occur during the water atomisation process.
Water atomised powders tend to be more irregular because the cooling rates in atomisation are 10 to 100 times higher than for gas atomised powders (Fig. 4). The product shape from water atomisation can be altered depending on the atomiser’s water jetting pressures and the jets’ angle to the melt. Changing these parameters can have significant effects on the process yield and on the overall shape of the powder particles.
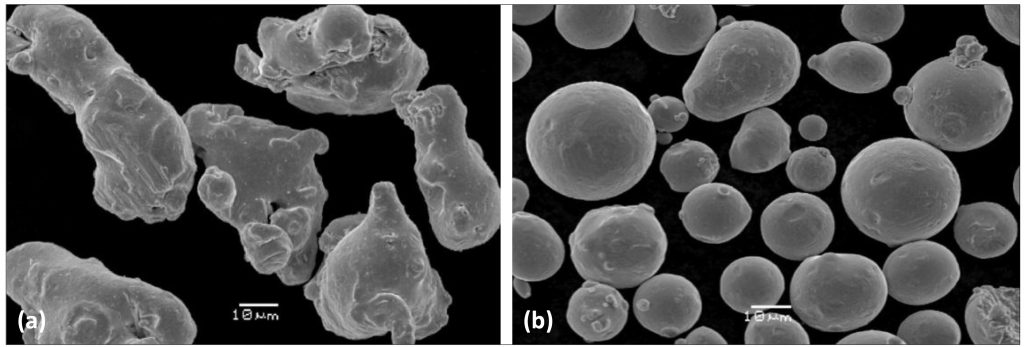
On the other hand, gas atomisation usually involves melting under a protective atmosphere or in vacuum. This limits the amount of oxidation and nitrogen pick up during the melting process. Due to the fact that oxidation is reduced during the process, there is no longer a strict manganese maximum dictated by the fear of severe oxidation of the metal. The compositional differences, discussed above, are reflected in the respective chemical analyses of the water and gas atomised powder batches reported in the study (Table 3). This table also includes the prescribed compositional specification for a cast 316L stainless steel.
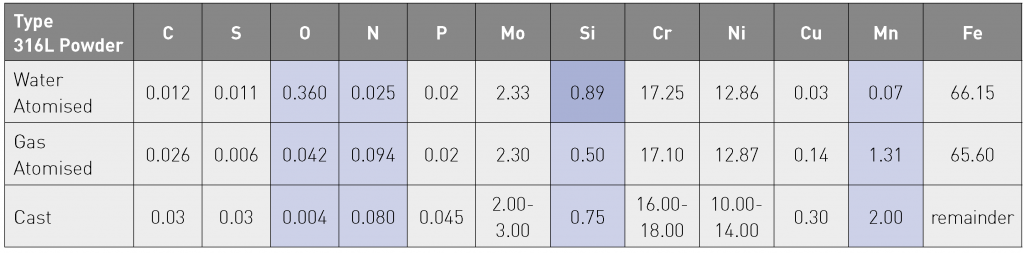
In gas atomisation, the gas to metal ratio is the primary parameter varied to control particle size. The process may also lead to gas becoming entrapped in the metal powder during atomisation, resulting in pores. This is especially noticeable when atomising with argon. Other than particle shape, the second factor influencing powder flowability is the particle size. Finer powders have much more difficulty in flowing due to higher surface areas and Van der Waal’s forces, compared with slightly coarser powders. The water atomised powder in this study, WA 316L, had a D50 of 24.40 µm and the gas atomised powder, GA 316L, had a D50 of 32.10 µm. In the context of both powder sphericity and particle size, the water atomised variant would be expected to show lower flowability than the gas atomised powder.
In Selective Laser Melting (SLM), final part density is correlated not only to the process laser power and scan speed, but also to the powder’s flowability. If the powder has the ability to spread well and in an unperturbed manner across each build layer, then the presence of porosity should be minimised. Figs. 5a and 5b show porosity images for both a gas atomised powder part and a water atomised powder part, built using a Renishaw AM250 machine. Even though the flow rate of the water atomised powder is different to that of the gas atomised powder, it has proved possible to modify the process parameters to print nearly fully dense bars of 99.84% relative density in each case. By optimising the laser power, hatch distance, exposure time and several other process parameters, the results produced with the water atomised 316L powder can be very comparable with those with the gas atomised powder.
In the reported study, tensile test-pieces were produced from the water and gas atomised powders using the process parameters optimised to achieve full density with each of these powder variants. Each build contained 12 tensile test-pieces with their gauge length in the X-Y plane. Table 4 presents the measured mechanical properties for the test-pieces, built by SLM from each of the powder variants, and, for comparison, the minimum property levels, quoted in the relevant standards for annealed 316L plate, sheet or strip (ASTM A240), for MIM 316L (MPIF Standard 35) and for cast 316L (CF3M), have been included.
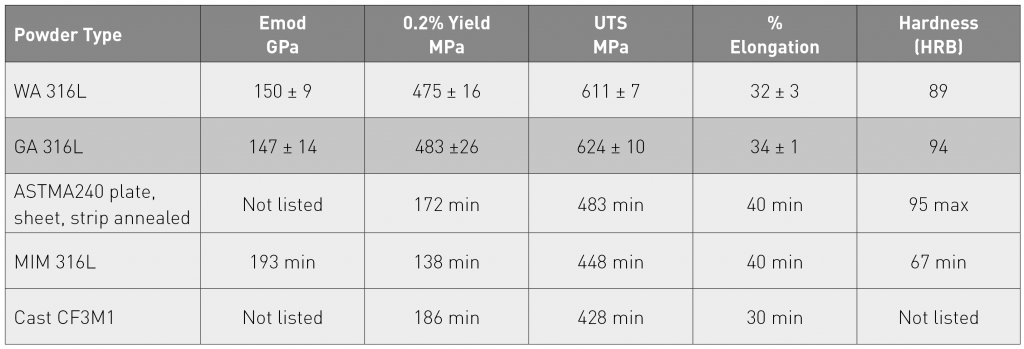
The tensile testing results for the SLM-built samples based on gas atomised 316L and water atomised 316L are very close, implying that water atomised powder can closely mimic the expected values from gas atomised powder.
The achieved strength levels for both SLM variants are substantially higher than the minimum values, quoted in Table 4 for 316L processed by other means. The authors have attributed this difference to the finer microstructures created by the high cooling rates in SLM. The higher strength levels in the SLM materials are accompanied by ductility levels that are lower than the quoted minimum levels for the other process variants, but, at over 30%, must still be regarded as being perfectly adequate for purpose.
Surface roughness can be an issue with SLM produced parts. Currently, however, this issue is not a hindrance to the use of water atomised powders because post processing is completed on all AM parts regardless of the powder type and its production route.
As stated previously, the SLM process parameters were optimised for the processing of water atomised powder. This alteration of the parameters, in comparison to the parameters for gas atomised powder, may lead to build times being slightly longer. It is currently not known how much the difference in time per part production would be, but, based on the changed parameters in this study, the difference cannot be so significant as to discredit the feasibility of utilising water atomised powder for AM.
The levels of powder flowability represent another area of difference between the two powder variants. In order to facilitate the use of water atomised powders, powder characterisation standards may need to be amended, as more sophisticated testing methods are introduced geared towards powders to be used in AM systems. One such method is the Powder Revolution Analyser. Currently, powders are being neglected for AM use, because of their flowability results from Hall and Carney flow-meters. This may, unfortunately, be eliminating powders, actually capable of being processed by SLM, because of the powder sizes and their perceived propensity to have poor flow.
The composition of the gas atomised 316L powder falls within the defined range in the ASTM standard (Table 3). However, the composition of the water atomised 316L powder is outside the range in terms of silicon content. However, the silicon contents measured in the SLM produced parts do not, in fact, vary significantly between the water atomised and gas atomised powder variants. The authors postulated that this could be explained by the formation of glassy oxides from silicon and oxygen.
The authors highlighted the issue that varying levels of chemical composition in water and gas atomised powders can play a significant role not only in mechanical properties, but also in corrosion resistance, a very important characteristic that could potentially compromise 316L’s utilisation in the medical industry. Biocompatibility applications are heavily reliant on the material’s corrosion characteristics, which are not only responsible for the structural stability of the medical component, but also have the potential to lead to the release of Ni, which has been identified as a severe cause of tissue inflammation. This issue, in relation to SLM built 316L parts, is the subject of continuing assessments by the authors.
References
[1] Microstructures and mechanical properties of Selective Laser-Melted metals with different processed powder, Naoyuki Nomura et al, as presented at the World PM2016 Congress, Hamburg, October 11-14 2016, and published in the Proceedings by EPMA, UK.
[2] Additive Manufacturing using water atomised steel powders, Simon Hoeges et al, as presented at the World PM2016 Congress, Hamburg, October 11-14 2016, and published in the Proceedings by EPMA, UK.
Author
Dr David Whittaker is a consultant to the Powder Metallurgy industry.
Tel: +44 (0)1902 338498
Email: [email protected]
Proceedings
The proceedings of the World PM2016 technical sessions and poster programme are published in digital format by the European Powder Metallurgy Association. For more information visit www.epma.com







