Stryker launches additively manufactured Monterey AL Interbody System
October 10, 2022
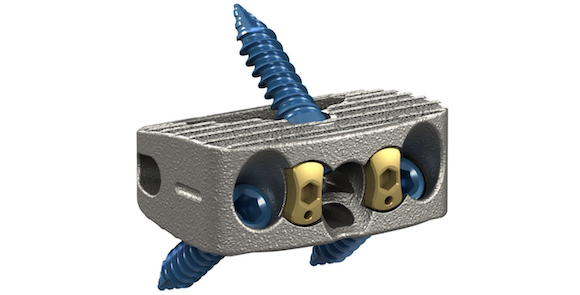
Stryker, Kalamazoo, Michigan, USA, has launched the Monterey AL Interbody System, a stand-alone interbody fusion device designed for anterior lumbar interbody fusion (ALIF). The device is built using AMagine, the company’s proprietary approach to Additive Manufacturing implants.
Monterey AL is constructed of both solid and porous structures, utilising Stryker’s proprietary Tritanium In-Growth Technology. This material is intended to mimic cancellous bone and provide an environment favourable to bone regeneration and fusion. Data has recently demonstrated that undifferentiated stem cells grown on Tritanium exhibited osteogenic Alkaline Phosphatase without requiring growth factor supplements.
“No one understands 3D printing like Stryker – the fact that they’ve been able to dial in the right mix of small, medium, and large pores in a reproducibly randomised matrix is incredible,” stated Bala Giri, MD, president and founder, Texas Neuro Spine Institute. “Their growing body of pre-clinical data – specifically the cellular findings published most recently – makes my decision to go with these products very straightforward. Our goal with any implant is spinal fusion, and Stryker has taken a very intentional approach to designing the Tritanium cages with this goal in mind.”
The Monterey AL’s strategically deeper and narrower cage footprints is said to allow surgeons to create indirect decompression by distracting the disc space posteriorly. These geometries are designed to both help prevent the cage from impinging posteriorly into the neural foramen and lessen the need to countersink the cage, thereby allowing for easy access to the anterior screw holes.
The medial attachment, multiple technique possibilities, and a wide variety of screwdriver options are also designed to facilitate clear visualisation of and easy access to the surgical site once the approach is complete and a retractor is in place.
“This is an exciting time for our division, as we continue to build momentum and expand our portfolio to bring new technology to our surgeon customers,” added Robbie Robinson, president of the Spine division, Stryker. “One of our goals as a medical technology company and an implant manufacturer is to complement clear visualisation and easy access with intuitive instruments and biologically inspired implant designs.
He concluded, “Monterey AL combines more than twenty years of expertise in the creation of porous materials using Additive Manufacturing with innovative implants and instruments that are designed to give surgeons the flexibility to use our system without having to alter their preferred technique.”
















