Osseus receives FDA approval for five new additively manufactured titanium spine implants
August 31, 2018

The Aries titanium implant has received FDA 510(k) clearance (Courtesy Osseus)
Osseus Fusion Systems, based in Dallas, Texas, USA, has announced FDA 510(k) clearance for Aries, its family of additively manufactured lumbar interbody fusion devices. The implants are constructed from titanium, optimised for bone fusion and biological fixation using PL3XUS, Osseus’ innovative and proprietary AM technology.
PL3XUS titanium technology utilises powder bed fusion, specifically Selective Laser Melting (SLM), to create 80% porous implants with increased bone packability and lower stiffness compared to competitive devices on the market. Aries implants are manufactured in 30-micron layers of titanium powder and sintered in solid, porous parts, in sequential layers. The implants then undergo a rigorous, proprietary post-processing cycle to optimise the device for clinical outcomes.
“We are thrilled to launch the Aries family of 3D-printed lumbar interbodies,” stated Eric Hansen, Co-Founder and CEO of Osseus. “The clinical benefits of 3D-printed titanium speak for themselves and Osseus is poised to capture market share in an exponentially growing industry like never before.”
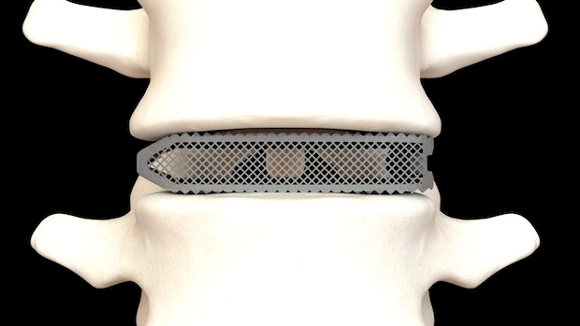
Osseus has launched five new titanium implants (Courtesy Osseus)
“In this industry, you’re perpetually considered the underdog if you’re private, self-funded company,” added Robert Pace, Co-Founder and CFO of Osseus. “We now have one of the most comprehensive portfolios of 3D-printed spine implants. Osseus is unique in our ability to bring surgeon-inspired implants to market quicker than any competitor, and our focus on R&D delivers unparalleled performance and quality to our surgeons and patients.”
This is the first FDA 510(k) clearance that Osseus has received for AM spine implants, and the approval effectively doubles the company’s product portfolio.
“This is just the first of many 3D-printed products that Osseus is working on,” stated Asher Breverman, Additive Manufacturing Engineer of Osseus. “We’re pushing the boundaries of design with new generative design software packages and surgeon-inspired products with our ability to work with surgeons like never before.”
















