H.C. Starck Tantalum and Niobium offers highly biocompatible alloys for medical applications
June 7, 2018
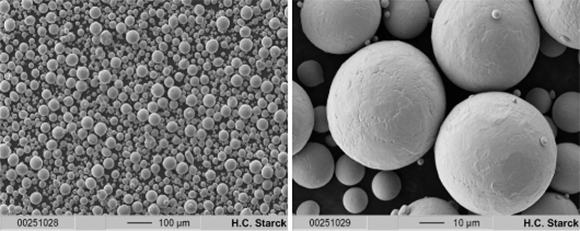
SEM images of gas-atomised AMPERTEC Spherical Ti-42Nb powders at 100 x (left) and 1000 x (right) magnification (Courtesy H.C. Starck Tantalum and Niobium GmbH)
H.C. Starck Tantalum and Niobium GmbH, Munich, Germany, has released a short report on the advantages offered by its biocompatible alloys for metal Additive Manufacturing. Among its materials product range, the company offers titanium, tantalum and niobium based alloys designed to meet the latest standards for medical technology.
Orthopaedic and dental implants are exposed to high mechanical loads during their lifetime. Though many of the materials currently used in Additive Manufacturing, including stainless steel and cobalt-chrome alloys, are able to cope with these mechanical stresses, there are some concerns regarding their release of toxic or allergenic elements which could result in inflammation of surrounding tissue and rejection of the implant.
An important prerequisite of all medical implants is their biocompatibility. As they are designed to remain in the body for a long period of time, the materials used in their production cannot have any effect on living organisms, regardless of the technology used to produce the implant. Proof of the biocompatibility of the materials used in medical implants is a primary approval criterion for their official regulatory approval.
“We are proud to be one of the trendsetters in the development of biocompatible alloys used in the fabrication of customised 3D printed implants,” stated Dr Melanie Stenzel, Head of Marketing & New Business Development at H.C. Starck Tantalum and Niobium GmbH.
Among its biocompatible alloys, the company offers AMPERTEC Spherical Ti-42Nb powders as well as tantalum alloys. These are produced using an electrode induction-melting gas atomisation process, and are said to be fully spherical with a negligible amount of satellites. The spheroidal shape of the powders improves their AM processability.
Because of their processing properties, AMPERTEC Spherical Ti-42Nb powders can be additively manufactured to almost full density (99.95%) using the Selective Laser Melting (SLM) process. This results in a low level of internal stress, meaning that thermal post-processing steps such as diffusion annealing or Hot Isostatic Pressing (HIP) are not typically required.
In addition, the phase composition of these materials is not affected by the laser melting process; similar to the atomised powders, the additively manufactured Ti-42Nb is pure β-phase. Additively manufactured parts also have a fine-grained microstructure with extremely homogeneous elemental distribution, and scanning electron microscopy with energy dispersive X-ray spectroscopy investigations is said to confirm that there is no segregation of Ti or Nb-rich phases in the powders.
Using mechanical analysis by means of tensile and compression tests, the company stated that the processed materials exhibit a combination of high elasticity and strength. Because Ti-42Nb is similar in its tensile elasticity to cortical bone, the use of this material is capable of reducing stress shielding between bone and implant, as well as the associated inflammation or implant loosening due to mechanical mismatches.
“The materials we have developed show excellent biocompatibility in comparison to commonly used alloys. Moreover, they have better mechanical properties, particularly regarding the higher elasticity, near to that of cortical bone, in comparison to conventionally applied implant materials,” concluded Stenzel. “Our new AMPERTEC Spherical Ti-42Nb powders represent the new generation of powders with excellent properties, best suited for these demanding medical applications.”