Additive Implants announces first surgeries with its titanium AM cervical spacers
July 15, 2022
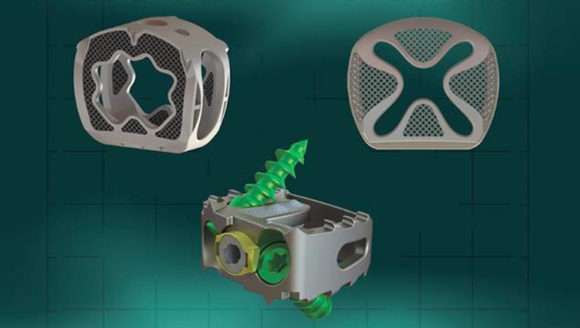
Additive Implants, Inc., a medical technology company focused on spinal surgery based in Phoenix, Arizona, USA, has announced the first implantations of its additively manufactured titanium cervical spacer, the SureMAX-SA™ Cervical StandAlone, the third system in the family of SureMAX Cervical Spacers.
Dr Pawel Jankowski of Hoag Hospital in Newport Beach, California, performed a series of procedures and stated he was pleased with his initial experience. Dr Jankowski felt the spacer was stable upon implantation and has been able to maintain initial surgical alignment during post-operative healing.
“The device endplates have a unique surface finish that will help facilitate the bone-implant interface. The large lateral window will allow me to visualise and follow the healing process. Instrumentation is extremely well thought out and one of the best I have seen, making the procedure to go very smoothly,” stated Dr Jankowski.
Dr Harvinder Bedi, Banner Boswell, Sun City, Arizona, has also utilised the system and commented, “The addition of the cervical standalone system really simplifies life for me and my surgical team. The implants are sterile-packed and, with one set of instruments for all three systems, I can easily change from standalone to a spacer and plate without opening additional trays.”
The SureMAX™, SureMAX-X™ and SureMAX-SA™ family of spacers from Additive Implants is said to offer one of the widest ranges of spacer sizes in the industry with options including 7-, 10-, and 14-degree lordotic angles; footprints of 12 x 14 mm, 14 x 16 mm, 15 x 18 mm, and 15 x 20 mm; and heights of 5 mm through 12 mm.
















