Titania nanotubes integrated into AM orthopaedic implants for localised drug delivery
November 22, 2021
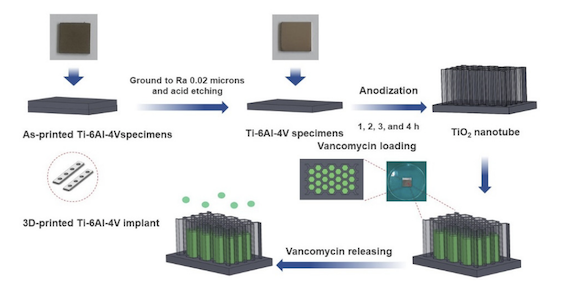
Recently published research in the Multidisciplinary Digital Publishing Institute (MDPI) journal Materials, ‘Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols,’ discusses the potential of additively manufactured titania nanotubes (TNTs) as targeted drug delivery systems in orthopaedic implants.
The move from simple casts and splints to internal implants like plates, screws and rods – typically made from steel, titanium, chrome, or cobalt – illustrates advances in supports for broken or fractured bones, but these implants carry risks: infection or rejection. In the worst-case scenario, these negative outcomes can result in limb loss or patient death. Despite these possible results, however, internal fixation is preferable for many patients, as, despite possible adverse effects, it commonly results in faster healing with a higher guarantee that bones will heal in the correct position.
To mitigate infection risks, antibiotics are used. Historically, however, it has been difficult to ensure that these target the implant itself when the antibiotics are delivered orally or intravenously. More localised delivery methods, such as antibiotic loading bone cement, offer a targeted option, but questions have been raised around its ability to release the antibiotics at sufficiently sustained levels. The recent paper suggests an alternative to this method: titania nanotubes, incorporated into the surface of bespoke additively manufactured implants.
Recent studies have reported that the use of TiO2 nanotubes coat of biomedical implant surfaces are biocompatible, well suited to tissue ingrowth and enables strong cell adhesion and proliferation. In this study, it was posited that TNTs also seemed a promising method for direct release of specific drugs into the site of infection.
The researchers on ‘Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols’ used an anodisation technique to synthesise TNT arrays on additively manufactured Ti6AL4V surfaces in order to observe and analyse the release the antibacterial vancomycin over a period of twenty-four hours.
SolidWorks 2020, CAD and CAE software from Dassault Systèmes, was used to design and model the Ti6Al4V plate implant 25 x 25 x 2 mm before it was additively manufactured using a Mlab Cusing 100R, a Laser Beam Powder Bed Fusion (PBF-LB) machine from GE Additive Concept Laser. The parts were produced using D50 micro Ti6Al4V powder by Meticuly Co Ltd, Bangkok, Thailand. During the anodisation process, this plate implant acted as the anode, while a commercial platinum plate (12 x 30 mm) from Umicore was the cathode. Under a controlled atmosphere, the Ti6Al4V plates were heated at 950°C for 2 hours in an electric furnace.
Prior to anodisation, the surface asperity of the Ti6Al4V plates was reduced by conventional grinding with 80 to 2000 grit paper and sonication within the acid solution, deionised water, and ethanol.
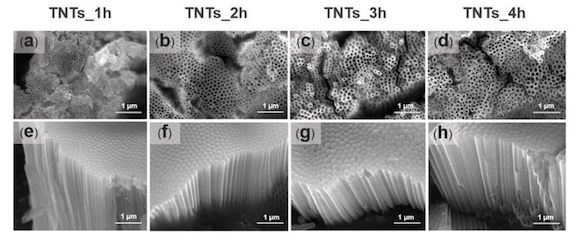
To achieve a nanostructured surface with orderly TNTs arrays with a very high aspect ratio, fluoride containing polyhydric alcohols – specifically, ethylene glycol, known for producing biocompatible and bioactive surfaces – was employed as the electrolyte. The ground specimens were immersed into the prepared ethylene glycol-based electrolyte containing a mixture solution of ethylene glycol 98 wt.%, ammonium fluoride (0.5 wt.%) and 1.5 wt.% deionized water for 1, 2, 3 and 4 hours. The electrolyte solution was continuously stirred with a magnetic bar at 100 rpm.
After manufacture, researchers studied the anodised TNTs using characterisation techniques such as field emission scanning electron microscopy (FESEM), contact angle meter, Fourier transform infrared (FTIR), an atomic force microscope (AFM), and X-ray photoelectron spectroscopy in order to note morphology, wettability behaviour, the interaction between ions of titania, pore size, length and surface roughness.
The results found that, by controlling certain parameters (e.g., electrolyte composition, voltage, atmospheric pressure, and anodisation time), TNTs were able to be enhanced sufficiently to act as suitable targeted drug release system when integrated into the surface of additively manufactured implants, thereby minimising the risk of infection.
The rough, nanostructured and nanoporous nature of the TiO2 formed on the Ti6Al4V surface is expected to facilitate the biocompatibility and osteointegration of the AM implant. The researchers have expressed their expectations that drug-loaded TNTs will serve as an alternative to the current systemic drug delivery approach used in treating infections.
The paper was written by H-thaichnok Chunate, Jirapon Khamwannah, Atchara Khamkongkaeo, Chiraporn Tongyam, Krittima Tumkhanon, and Torlarp Sitthiwanit of the Department of Metallurgical Engineering, Chulalongkorn University, Bangkok, Thailand; Abdul Azeez Abdu Aliyu, Chedtha Puncreobutr, and Boonrat Lohwongwatana of both the Department of Metallurgical Engineering and the Biomedical Research Center at Chulalongkorn; Thanawat Phetrattanarangsi and Dechawut Decha-umphai of the Department of Metallurgical Engineering at Chulalongkorn and the Biomechanics Research Center at Meticuly Co. Ltd., Bangkok; Saran Tantavisut of the Orthopaedic Department at Chulalongkorn; Pharanroj Pongjirawish of the Biomechanics Research Center at Meticuly; and Theerapat Chanamuangkon of the Biomaterial Testing Center at Chulalongkorn.
‘Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols’ is available in full here.
















