Osseus’ AM spinal interbody system receives FDA clearance
May 11, 2022
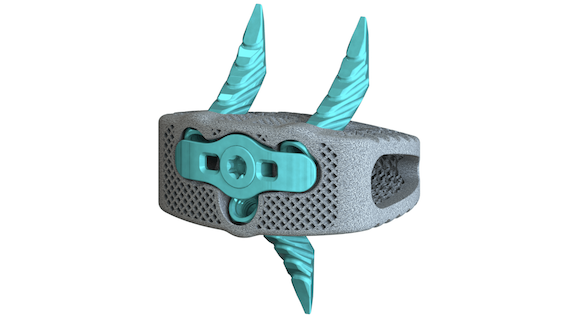
Osseus, Dallas, Texas, USA, an innovative spinal solutions company, has announced the FDA 510(k) clearance and launch of the Pisces™-SA Standalone ALIF Interbody System. The Pisces-SA interbody is produced via Additive Manufacturing and can be used with both bone screws and alternative fixation bone anchors allowing for increased intra-operative flexibility.
Biomechanical testing is reputed to have proved that the Pisces-SA anchors provide better expulsion resistance than the competition and perform comparably with traditional screw-based standalone ALIF constructs in stabilising injured spinal segments. The Pisces-SA is said to be the first of its kind to provide this level of expulsion resistance and segmental stabilisation using an alternative fixation method.
“I’ve used screws […] and I’ve used the blade-type constructs, but I’ve never used a device where you could make that choice intraoperatively,” commented Dr Michael Hisey, an orthopaedic surgeon with Texas Back Institute in Plano, Texas. “I really like that ability to make my decision at the time.”
This platform integrates a highly porous additively manufactured interbody with anatomical morphology designed for full osseointegration with streamlined instrumentation to facilitate a minimally invasive approach. Osseus believes the Pisces-SA sets the new standard for Standalone ALIF devices.
“Receiving FDA approval for the Pisces-SA is the culmination of relentless work from our R&D department and surgeon design team. We feel this product launch will solidify Osseus as one of the leading innovators in the spine industry,” stated Rob Pace, founder and CEO of Osseus. “Since our inception, we have pushed the envelope creating minimally invasive products to help simplify and streamline procedures. This product hits that mark, and we are excited to introduce it to the market. This is a special day for our company and for the team of surgeons who have provided extensive input on this groundbreaking product.”
















