Nexxt Spine adds fifth Concept Laser Mlab Additive Manufacturing system
April 29, 2019

Nexxt Spine has added its fifth Concept Laser Mlab system (Courtesy Nexxt Spine)
Nexxt Spine, Noblesville, Indiana, USA, an independent medical device company which designs and manufactures spinal solutions, has invested in its fifth Concept Laser Mlab system, supplied by GE Additive. The company was established in 2009 and initially produced spinal screws, rods, and plates using conventional subtractive manufacturing techniques. Its first investment in metal Additive Manufacturing was the acquisition of a Concept Laser Mlab 100R in 2017.
“We used the first Mlab primarily for R&D purposes, but we soon realised that further investment in Additive Manufacturing technology could add value not only to our overall growth strategy, but also at a clinical application level with the ability to develop implants with very intricate micro-geometries that could maximise healing. Over the past two years, we have made a seamless jump from R&D to serial production and in doing so have significantly accelerated the time from concept to commercialisation,” stated Alaedeen Abu-Mulaweh, Director of Engineering at Nexxt Spine.
The investment in Concept Laser Mlab systems has reportedly enabled Nexxt Spine to take ownership of the entire design, production and distribution process in-house, eliminating the need for contract manufacturers, thereby accelerating the speed of development and commercialisation. In recent years, the company has been focused on the design and development of spinal fusion implants that incorporate interconnected micro-lattice architectures with the goal of promoting osteoconduction, osseointegration, and boney fusion.
The Nexxt Matrixx® System, which Nexxt Spine launched in 2017, is a collection of porous titanium spinal fusion implants that interweave highly differentiated surface texturing technology with additively manufactured cellular scaffolding. The company reported that, while other medical manufacturers have used Additive Manufacturing to develop devices that directly mimic bone’s trabecular geometry, Nexxt Spine chose instead to blend cellular porosity, inspired by natural bone biology, with core engineering fundamentals to develop structurally sound devices, optimised for fusion.
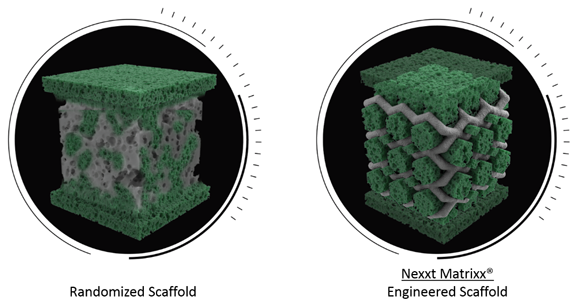
A typical implant scaffold and Nexxt Spine’s Nexxt Matrixx engineer scaffold (Courtesy Nexxt Spine)
Abu-Mulaweh added, “Titanium – porous or otherwise – is physically incapable of biological remodeling, so using Additive Manufacturing to directly mimic the structural randomness of bone doesn’t make a whole lot of sense. Rather than simply looking like bone, Nexxt Matrixx was designed with functionality in mind to fulfill our vision of actively facilitating the body’s natural power of cellular healing.”
“We are seeing ongoing adoption of Additive Manufacturing in the orthopaedic industry and an exciting shift from research and development to serial production. Early innovators like Nexxt Spine are scaling up and there is a significant increase in production volumes,” commented Stephan Zeidler, Senior Global and Key Accounts Director for the medical sector at GE Additive.
Nexxt Spine stated that, with design, manufacturing and distributions functions in-house, combined with the shift to serial Additive Manufacturing production, the company is well-placed to service and scale, as needed, to meet the growth in demand for spinal fusion devices.
“Nexxt Spine is another great example that shows the power of our Mlab machine, which is proven to be an easily accessible machine for research & development, with the capability to be a reliable, scalable and modular production machine at the same time,” added Zeidler.
Abu-Mulaweh further added, “Like I said, Additive Manufacturing is absolutely booming. It is driving our business and innovation strategy forward and our design team is actively developing and testing new applications, parameters and surgical devices to target new markets. We are excited for what the future holds for us.”
















