Medical implant maker Rejoint selects GE Additive Arcam EBM Q10plus
June 17, 2020

Rejoint, a medical implant manufacturer headquartered in Bologna, Italy, has adopted GE Additive’s Arcam EBM Q10plus Additive Manufacturing machine into its production. The addition of this machine is expected to enable the company to introduce mass-customisation and therapy personalisation through a combination of Electron Beam Powder Bed Fusion (EB-PBF), referred to by GE Additive as Electron Beam Melting (EBM), and computerised analysis of intraoperative and post-operative data collected through IoT-connected sensorised wearables.
Rejoint explained that the market for knee implants is now estimated at around five million implants per year globally. In advanced markets, as of 2011 the number of surgical procedures per year was 150 per 100,000 inhabitants, with peaks of 250 in some markets such as Austria and Switzerland.
Patient-specific arthroplasty
The knee arthroplasty market, until recently, consisted solely of standard prosthetic systems, with a limited range of sizes available. Correct and precise sizing and positioning are critical factors for the success of this type of intervention, which is now clinically routine, but still variable in terms of success, stated the company.
Knee joints have to withstand point loads that can reach levels of over 300 kg, as even minimal dimensional changes between the patient’s bone elements and an implant can cause pain and inflammation. For the patient, over- or under-sizing can result in constant awareness of the presence of an artificial joint, as well as leading to muscle and ligament decay.
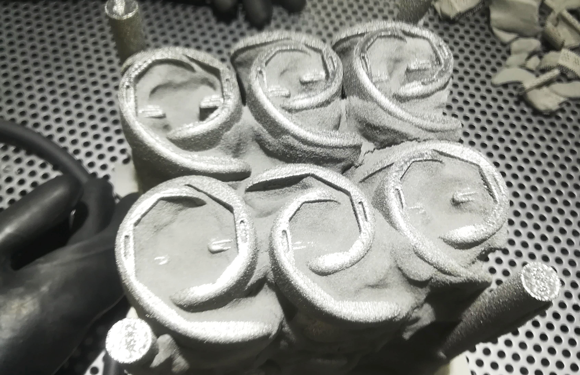
From the patient’s perspective, there has been a significant change in the criteria for choosing an implant solutio. While some older patients might rely entirely on their doctor to make that choice for them, younger patients may go online to become better informed on the solutions and treatment paths available to them, and choose a surgeon based on the information they gather.
Additive Manufacturing and data-driven customisation
Founded in 2015 by an experienced orthopaedic team, Rejoint has recently entered the knee arthroplasty market and explains that both Additive Manufacturing and artificial intelligence are integral to its growth strategy.
“When it came to Additive Manufacturing, we were initially undecided about the most suitable solution for personalised cobalt-chrome prosthetics and were evaluating DMLM [LB-PBF] and EBM [EB-PBF],” commented Gian Guido Riva, CEO at Rejoint. “Both modalities, in fact, offer a good level of resolution and quality, but we ultimately opted for the GE Additive Arcam EBM Q10plus system.”
“The knowledge and industrialisation support that GE was able to provide us and the professional experience of their local team here in Italy also informed our decision,” Riva continued. “At the moment, ours is still the only solution for additively manufactured knee prostheses in cobalt-chrome to be certified and introduced to the market.”
In order to produce the additively manufactured prosthesis, Rejoint starts by 3D modelling the patient’s CT scan. Sophisticated artificial intelligence algorithms are then used to analyse the images and identify the most suitable size for each specific case. Artificial intelligence is used to compare the unique anatomy of a patient on several thousand prosthetic dimensions, each with as many dimensional variables in specific areas of the implant.
The surgeon is then offered the optimal configuration for positioning both the prosthetic components and for simulating the operation. This analysis forms the basis for the production of the prosthesis and for patient-specific tools for the planning of the intervention – which is carried out with the support of computer-aided surgery tools.
Riva added, “Having all this data made us realise that we could link it to the information recorded during the operation. And in turn, this data could still be further improved upon if we could collect through the use of wearable devices (such as sensorised headbands and socks), both pre- and post-operative measurements, on how the patient loads their limb or bends their knee until postoperative evaluation questionnaires have been completed.”
This approach, which is now part of Rejoint’s offering, is said to make it possible to identify a series of correlations that trace the whole process, from the interactive preoperative planning right through to the rehabilitation phase.
“By 2022, we will have the complete data of thousands of cases available,” Riva explained. “This will provide us with an unparalleled wealth of application information, in terms of completeness, in the sector. Despite the sale of millions of pieces, there is little or no information on what happens post-sale.”
Professor Maurilio Marcacci, Head of the Joint Knee Reconstruction Centre at Humanitas Research Hospital in Milan, Italy, who reportedly performed the first implantation of a Rejoint product, stated that the initial application of this technology has achieved a high degree of patient satisfaction, which he states is unprecedented in his many years of experience.

Future developments
Rejoint is currently in the process of obtaining US Food & Drug Administration (FDA) 510(k) clearance, which is expected in the first half of 2021. Certification will mean access to the US market, which accounts for 62% of the world market for orthopaedic devices and more than 70% of the value of the global market for knee implants.
The company is working with GE Additive to reduce powder-based production costs, focusing on the reduction of cycle times and the optimisation of parameters – including through the development of remote production control stations. For younger or less acute patients, Rejoint is also said to be developing a single-compartment prosthetic system with a minimally invasive design and robotic surgical technology.
“The key element is an increasingly close and direct relationship between company and patient. This will further increase the degree of post-operative satisfaction. We are at the beginning of a revolution in the field of knee implants. Rejoint’s work in adopting additive technology will allow for more personalised procedures and higher levels of long-term patient satisfaction,” concluded Riva.
















