Mantiz utilises Eplus3D’s EP-M260 metal AM machine for titanium spinal cage production
September 6, 2021
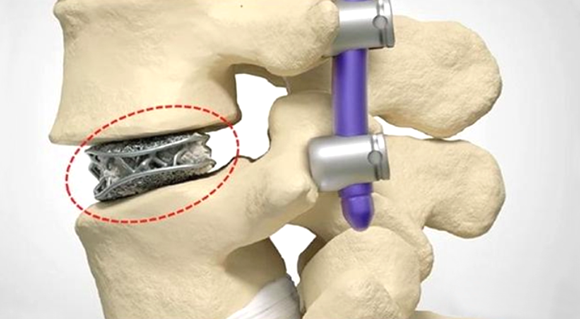
Eplus3D, Hangzhou, China, reports that medical device company Mantiz, based in Daegu, South Korea, has used the company’s EP-M260 metal Additive Manufacturing machine to produce additively manufactured titanium cages for use in spinal implant surgery.
Established in 2015, Mantiz develops and manufactures spinal implants and bio-related products with the aim of helping patients with spinal diseases achieve a pain-free life. The company has obtained KFDA (Korean Food and Drug Administration) certification and is listed on the HIRA (Health Insurance Review and Evaluation) list in South Korea.
Mantiz began developing additively manufactured cage implants in 2018, and, in May 2019, the company launched its Panther cage system for PLIF (Posterior Lumbar Interbody Fusion) surgery. This process allows the company to use its additively manufactured cage implants without outsourcing the production process – saving clients time and money.
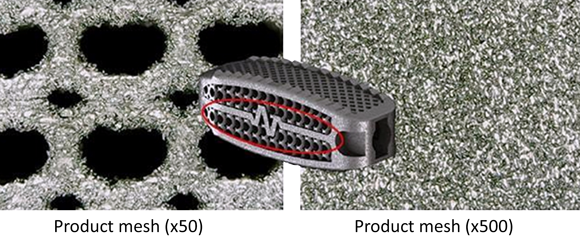
The EP-M260, an industrial metal AM machine that uses the Laser Beam Powder Bed Fusion (PBF-LB) process, features a 266 x 266 x 390 mm build chamber that can reportedly be used to additively manufacture more than fifty individual implants in one structure.
The size, material, shape and porosity are essential to the effectiveness of the implant. Upon transplantation, the bone and surrounding tissue begin to fuse with the implant, creating a strong structure in the patient’s spine.
Hongwon Yoon, CTO of Mantiz and the inventor of the Panther AM cage system, stated, “We have completed the development of a more improved titanium 3D printed cage implant using the EP-M260 3D metal printer. Mechanical test results prove the safety and functionality of the implant. 3D printing the average closed pore ratio of the solid titanium component is 3%, which promotes the binding of the protein for bone fusion to the mesenchymal stem cells.”
















