Lincotek Medical celebrates over 800,000 implantables with AM
October 28, 2022
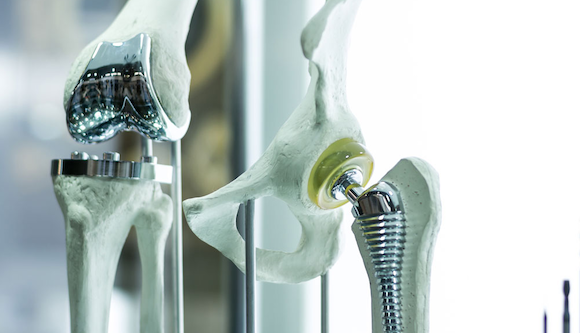
Lincotek Medical, headquartered in Trento, Italy, recently celebrated having produced over 800,000 implantable devices since it began Additive Manufacturing in 2006. From Italy, Lincotek focuses on the medical sector; Switzerland, industrial applications; while the US hosts the organisation’s Additive Manufacturing plants and related R&D services.
Lincotek additively manufactures with titanium alloys (e.g., CpTi and Ti6Al4V), cobalt alloys (e.g., CoCrMo), steel (e.g., 17-4 SS and AlSl316L), nickel alloys (e.g., HastX, IN625, IN718, IN738 and IN939) using Electron and Laser Beam Powder Bed Fusion (PBF-EB and PBF-LB).
The company offers a variety of post-processing solutions, including thermal treatments, machining, coating, surface finishing and from final cleaning to final certification.
In the medical field, Lincotek’s plants are ISO 13485 certified and release implantable medical devices (CE and FDA) and its IGT/Aviation plant’s main production facilities are certified AS9100/NADCAP.
















