GE Healthcare supports development of AM medical components
April 6, 2017

Stephen Abitz, GE Healthcare’s Additive Process Leader, holds a tungsten collimator prototype (Courtesy GE)
GE Healthcare’s Advanced Manufacturing & Engineering Center, Waukesha, Wisconsin, USA, is supporting the development of Additive Manufacturing processes for medical components at seventy GE factories worldwide, the company states. According to GE, the development drive is aimed at the creation of better medical imaging technologies and quicker manufacturing processes for key medical parts and components, as well as an additive-focused training environment for new engineers.
Metal AM development began at GE Healthcare in 2013, with the first complete medical component – a collimator – produced in 2015. Resembling a fine metal honeycomb, a collimator is installed in X-ray and CT scanners to filter out unwanted signal noise and keep imaging crisp. The development of an additively manufactured collimator lowered overall component production cost by 40% and reduced the number of parts to two, GE reports, in comparison to the hundreds of externally sourced parts used to produce traditional collimators.
Though this project took two years from development to completion, the next metal AM prototype took just weeks to design and print. “When you figure it out, it’s much easier to do the next one,” explained Stephen Abitz, GE Healthcare’s Additive Process Leader. “You are building off your knowledge and not starting from scratch.”
Among GE Healthcare’s other new projects are the Direct Write Printer, a highly specialised AM machine which the company reports is capable of ‘writing’ silver and other metallic glyphs on parts for ultrasound machines and other healthcare equipment. These ‘glyphs’ – electronic circuits for wireless antennae, RFID tags and other sensors – are intended to cut out the cost of cabled circuit systems. The Direct Write Printer mixes nitrogen gas with droplets of copper, silver, gold and semiconductor inks to form an aerosol, which is then deposited by jet onto the required surface.
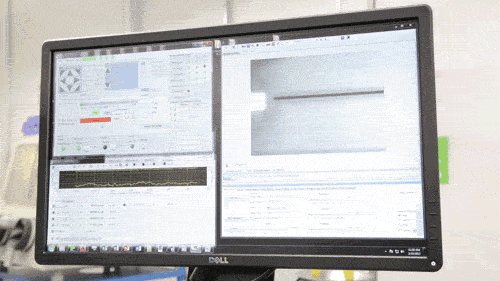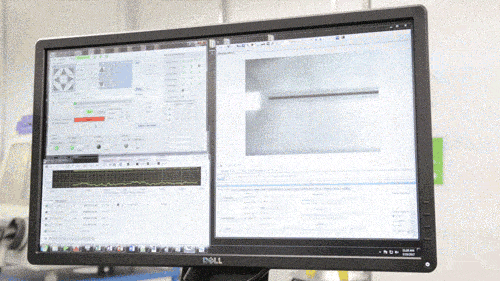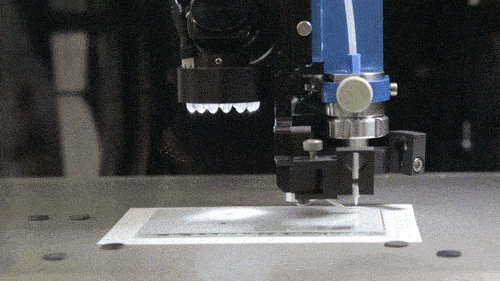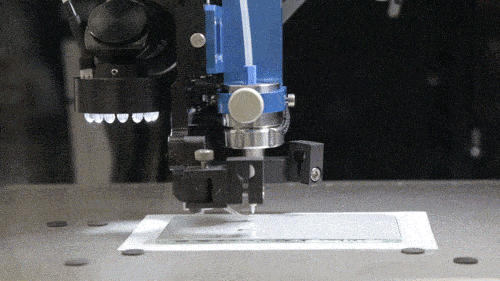
The Direct Write Printer at work (Courtesy GE)
In the company’s Life Sciences segment, Additive Manufacturing has been used to develop custom moulds for the casting of chromatography columns – pharmaceutical research equipment used to filter the correct proteins and RNA from bioreactors in the development of biopharmaceuticals used to treat diabetes, MS and cancer. By developing an Additive Manufacturing method for filter moulds, GE Healthcare reports that it has reduced the manufacturing cost from $50,000 for the traditional process, which relies on external suppliers and is extremely time-consuming, to less than $1,000 per mould, created in-house over a few days.
In collaboration with GE’s Center for Additive Technology Advancement in Pittsburgh, Pennsylvania, USA, GE Healthcare is also working on the development of additively manufactured moulds for parts used in MRI and mammography machines.
Jimmie Beacham, GE Healthcare’s Chief Engineer for Advanced Manufacturing, stated, “With [the speed of] Additive Manufacturing, if your design isn’t perfect, you fix it and print it again. You can go through several design iterations in a single week. That’s never been possible before.”
GE Healthcare’s focus on AM mirrors the spread of additive technologies across the GE group, with GE Aviation, Power and Oil & Gas segments already having begun to additively manufacture fuel nozzles and other components. In 2016, GE acquired majority stakes in AM companies Arcam AB and Concept Laser and launched a new AM segment of its own – GE Additive. Jeff Immelt, GE’s Chairman and CEO, has stated that he believes Additive Manufacturing represents a $75 billion opportunity for the group.
















