4WEB Medical receives 510k clearance for new integrated cervical plate
October 5, 2023
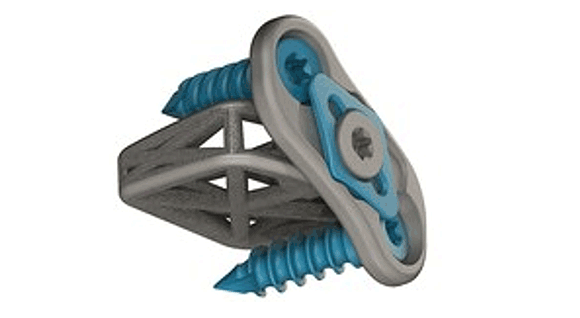
4WEB Medical, an orthopedic implant company based in Frisco, Texas, USA, has obtained 510(k) US Food and Drug Administration clearance to market its new Cervical Spine Truss System (CSTS) Integrated Plating Solution, as well as two cervical interbody line extensions. 4WEB Medical’s proprietary truss implant platform was reported to be the first 510(k) cleared additively manufactured implant.
Jonathan Hires, Director of Research and Development commented, “The CSTS Integrated Plating Solution provides an additional stand-alone treatment option for 4WEB’s surgeon customers. With two product launches in Q3 and regulatory clearance for the integrated plate, we have built significant momentum towards completing the company’s comprehensive cervical portfolio by the end of the year.”
The CSTS Integrated Plating Solution clearance is the latest achievement in a series of milestones for the company. This includes the successful launch of a non-integrated cervical plating solution and a second-generation cervical interbody fusion device in the third quarter. Furthermore, there are plans to introduce an integrated anchor fixation system in early Q4. This expanded product suite offers a range of cervical fusion constructs to meet different anatomical requirements. With these advancements and ongoing product development, 4WEB has positioned itself as a leader in the cervical interbody fusion market.
“The launch of the CSTS Integrated Plating Solution builds on an already robust product portfolio. We are excited to launch several new cervical products before the end of the year and look forward to capitalising on this success with significant growth in 2024,” Geoff Bigos, Vice President of 4WEB Medical’s Spine Division, added.
Download Metal AM magazine

















