Stryker receives FDA clearance for metal additively manufactured lumbar cage
March 14, 2018
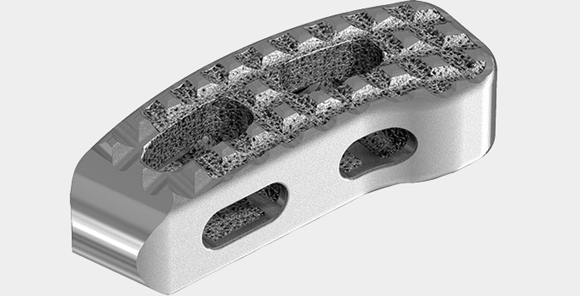
Stryker’s Tritanium TL Curved Posterior Lumbar Cage with porous titanium surface (Courtesy Stryker)
Stryker’s Spine Division has received 510(k) clearance from the US Food and Drug Administration (FDA) to begin commercialising its metal additively manufactured Tritanium® TL Curved Posterior Lumbar Cage, an interbody fusion cage intended for use as an aid in lumbar fixation. The cage comprises a hollow implant with what Stryker states is a unique configuration of both solid and porous structures, built using AMagine™, the company’s proprietary approach to implant creation using Additive Manufacturing.
The cage incorporates Stryker’s Tritanium Technology, a highly-porous titanium material which mimics the microstructure of trabecular or ‘spongy’ bone tissue to inspire osseointegration (bone in-growth) and biological fixation. The Tritanium material is also said to have the potential to wick or retain more fluid in comparison to traditional titanium material.
According to Stryker, the Tritanium TL cage is designed to complement the company’s preexisting Tritanium PL cage, a posterior lumbar cage which is not curved. Together, the devices are said to offer alternative posterior lumbar solutions for spinal surgeons. “The Tritanium TL Cage is the latest addition to our highly successful Tritanium portfolio, which has been embraced by spinal surgeons nationwide,” explained Bradley Paddock, President of Stryker’s Spine Division.
“The TL cage is accompanied by a new Anterior Placement System that is designed for versatility and procedural flexibility. From instrumentation ergonomics and visualisation, to a simplified technique with tactile feedback, Tritanium TL’s Anterior Placement System and cage design redefine implant steerability for surgeons.”
The Tritanium TL Cage is expected to be available to surgeons in the second quarter 2018.
















