Nexxt Spine’s latest additively manufactured spinal implant receives FDA clearance
August 13, 2019
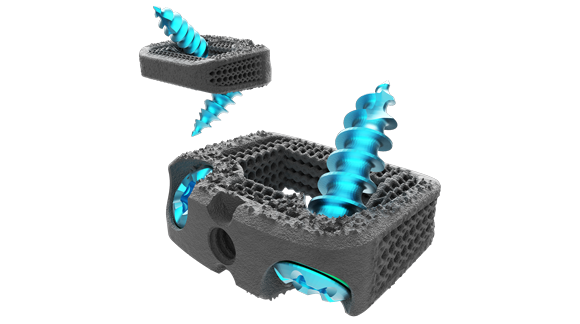
Nexxt Spine LLC, a medical device manufacturer based in Noblesville, Indiana, USA, has reported that its Nexxt Matrixx® Stand Alone Cervical System, which was additively manufactured on a GE Additive Concept Laser Mlab system, has received FDA 510(k) clearance and can now enter the market.
The new cervical system is a stand-alone anterior cervical interbody fusion system intended for use as an adjunct to fusion at one or two contiguous levels (C2-T1) in skeletally mature patients for the treatment of degenerative disc disease. It is intended to be used with the bone screw fixation provided and requires no additional fixation.
“This enhancement of the Nexxt Matrixx portfolio was the next natural progression for Nexxt Spine,” stated Andy Elsbury, President, Nexxt Spine. “With patient care always top of mind, we strive to develop end products that surgeons prefer and hardware patients can count on. Our Stand Alone Cervical is no exception and will showcase the propensity of Nexxt Matrixx technology to facilitate the body’s natural power of cellular healing for fortified fusion.”
















