Metal Additive Manufacturing enables device with potential to cure Parkinson’s disease
March 8, 2019
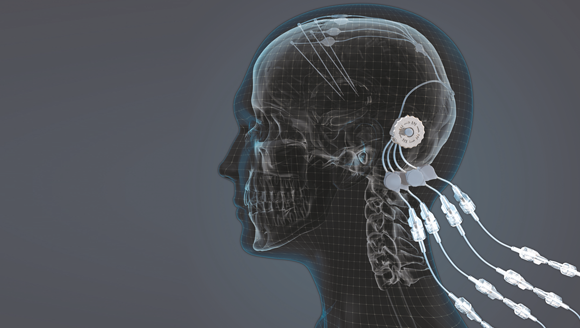
The new drug delivery system, manufactured by Renishaw for the North Bristol NHS Trust, offers promising results for the treatment of Parkinson’s disease (Courtesy Renishaw plc)
Renishaw plc, Gloucestershire, UK, has produced a titanium port by metal Additive Manufacturing for use in a ground-breaking clinical trial. Manufactured on behalf of the North Bristol NHS Trust, the device enables the precise delivery of a new drug candidate, Glial Cell Line Derived Neurotrophic Factor (GDNF), directly into the brain of individuals with Parkinson’s disease, with the aim to regenerate dying dopamine brain cells and thereby improve symptoms.
Renishaw worked with Prof Steven Gill, Consultant Neurosurgeon, to manufacture the new drug delivery system, which reportedly offers a practical method to bypass the blood-brain barrier. During the trial, forty-two patients had the AM titanium port embedded into their skull, through which GDNF was delivered via micro-catheters to the putamen, a critical region of the brain for motor function. The device was implanted using the Renishaw neuromateTM surgical robot, which positioned four catheters into the brain.
The trial was funded by Parkinson’s UK with support from the Cure Parkinson’s Trust, in association with the North Bristol NHS Trust. Results announced in February 2019 were said to show that the drug delivery system performed effectively and reliably, and a similar device developed by Renishaw called neuroinfuse™ is now being used in other clinical trials.
The results of the trial were aired on television in the UK as part of a documentary, The Parkinson’s Drug Trial: A Miracle Cure?, on BBC Two. Paul Skinner, General Manager for Neurological Products at Renishaw, stated, “It has been a privilege to work alongside the study team and with the participants in this ambitious trial. We are very encouraged that there were changes in the brain scans, demonstrating that GDNF is having an effect, and that the delivery system achieved precision administration of drugs into the brain.”
“This provides great potential for using the drug delivery system, being developed by Renishaw, for future Parkinson’s studies and experimental treatments for other neurodegenerative diseases and brain tumours,” he concluded.
Prof Gill commented on the results, “This is a significant breakthrough in our ability to treat neurological conditions such as Parkinson’s, because most drugs that might work cannot cross from the bloodstream into the brain due to a natural protective barrier. Even at a low dose we have seen evidence of patient improvement, which is incredibly encouraging.”
















