Additively manufactured cranial implants receive EU approval
November 12, 2014
Slovakian company CEIT Biomedical Engineering has utilised Additive Manufacturing technology from EOS to provide doctors with precise bone replacement material. The process has received registration from the Slovakian State Institute for Medicinal Control for the manufacture of implants for cranial, facial and jaw applications and this has resulted in official EU-wide approval, states EOS.
A cranial implant must fit precisely to ensure long-term success of a surgical operation and ensure that the patient is able to live without discomfort after an accident or serious illness. Conventional subtractive metalcutting has traditionally been used to manufacture such custom implants. However, certain shapes are impossible to manufacture sufficiently accurately, necessitating manual work by the surgeon during the operation. Moreover, the process is limited in what it can achieve and tends to be lengthy and expensive.
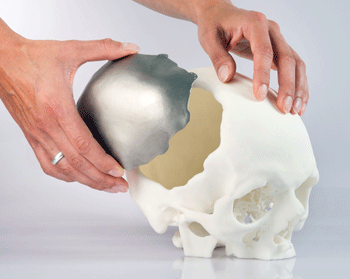
CEIT Biomedical Engineering is using an EOSINT M 280 to
produce bespoke and standard implants from Ti-6Al-4V
powder
CEIT Biomedical Engineering, a spin-off company of the Technical University of Košice, was created to promote Additive Manufacturing in the medical sector. Associate Professor Radovan Hudak, Managing Director, stated, “We wanted to explore the potential of Additive Manufacturing technology for implantology and at the same time develop solutions that were both helpful to patients and economical.”
“It was necessary to explore the limits of the technology and its potential as well as to discover the optimum process and find a suitable material. Precision, reproducibility and surface quality were all high on the list of requirements, along with a production process that was as free as possible from production errors. The main aim was then to acquire state certification for cranial, jaw, and facial bone implants,” stated Prof Hudak
The CEIT team took a year to explore the market and to analyse which Additive Manufacturing systems were available, eventually deciding on EOS as its technology provider and its titanium alloy as the starting material.
Prof Hudak added, “We are able to meet the technological criteria and manufacture extremely thin walls with the necessary uneven surface geometries. In addition, it is possible to introduce cavities into the implants, such as shapes comprising complex hollows or canals. Lattice structures have become a key element in the scope of the possibilities.”
For manufacturing the first implant, the task was to produce a piece of replacement cranium approximately 15 cm across from titanium Ti-6Al-4V, a biocompatible standard alloy in medical technology with excellent mechanical characteristics.
The team used the results of a computed tomography examination of the patient. Data transformation to a CAD program and design and manufacture of the implant all took place at CEIT. The 1.5 mm thick implant weighed 63g. A notable feature was the hollow lattice structure, which promotes in-growth of bone tissue and allows the integration of micro-sensors for recording medical data.
CEIT is reported to be producing bespoke implants as well as undertaking series manufacture of standard implants using EOS additive manufacturing technology.
Subscribe to our FREE e-newsletter
Useful links: News | Articles | Introduction to metal Additive Manufacturing
















