Lincotek Medical expands US facilities after further investment
March 3, 2021
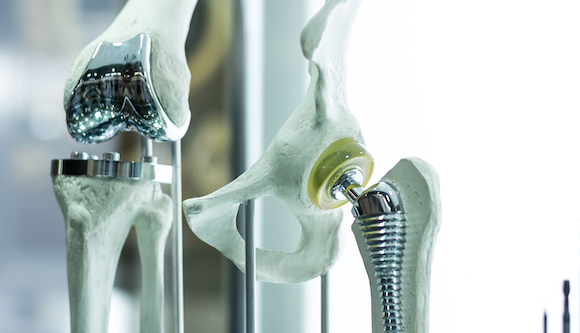
Lincotek Medical, headquartered in Trento, Italy, is continuing its global medical investment programme to expand its facilities and help OEMs meet the rising demand for orthopaedic implants through new technologies, automation and workforce training.
The latest step has been focused on the expansion of its Bartlett plant in the greater Memphis, Tennessee, USA, area, where the company has recently doubled its manufacturing space to around 2,300 m2 (25,000 ft2), with a new industrial workspace.
Lincotek Medical is reported to be investing heavily in AM. As a company already known for its use of medical devices with AM technology, and with several additive machines already in production in Bartlett, Tennessee, Lincotek Medical is further increasing its Laser Beam Powder Bed Fusion (PBF-LB) capabilities in the US. It has validated its AM process, in line with FDA (Food and Drug Administration) guidance, and invested in a dedicated AM team, supporting OEMs managing complex supply chains with new solutions. An in-house metallurgical laboratory supports production, maintaining high standards of quality throughout the operation.
“If it is about additive technology for medical devices,” stated Francesco Bucciotti, Managing Director, USA. “Lincotek Medical is the go-to partner for OEMs, ensuring reliable and cost-efficient additive device manufacturing. Starting with Additive Manufacturing of medical devices in 2006, the company has profound additive development and production experience, significantly reducing time to market for its customers.”
The company has also significantly increased its coating capacity with the purchase of two new pieces of automated equipment, a CAPS Intercooler and an Air Plasma Spray (APS), along with an automatic sandblasting machine, all created by Lincotek’s Equipment Division. With the installation of a new CAPS Intercooler – a proprietary technology, with advanced porous coatings and unique material performance – Lincotek Medical can now rely on two CAPS Intercoolers in the US, two in the EU and two in Asia-Pacific. This will allow replicable consistency-of-quality in coatings globally through the same process control, ennsuring optimal quality performance.
The coating investment is completed by the new APS line, which is based on Lincotek’s twin approach, allowing for movement from one line to another, achieving identical coating parameters, by moving the production programmes.
The validation of Lincotek’s range of orthopaedic device coatings is supported by multiple master-files with the FDA. All process inputs are monitored and recorded, assuring quality and supporting OEMs in auditing.
The company hopes that its expansion of coatings and additive capacity will enable market growth, while significantly increasing flexibility and production capacity. Orthopaedic OEMs will benefit from a reduced time to market and stronger global risk mitigation capabilities.
Gennaro D’Andrea, General Manager, Lincotek Medical Division, concluded, “Despite the unique challenges in 2020 posed by COVID-19, the Lincotek Medical team has continued its strong support for OEMs with multiple successful product launches for both coatings and AM. As the industry demand for orthopaedic implants continues to rise, Lincotek Medical is poised and ready to support the OEMs with the capacity, technology and experience required to produce high-quality products.”
















