3D Systems receives FDA 510(k) clearance on its material options for its Virtual Surgical Planning technology
October 1, 2020
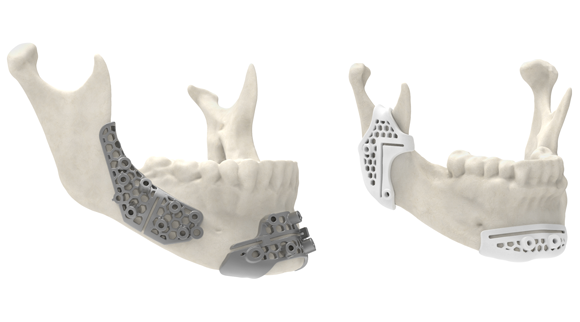
3D Systems Corporation, Rock Hill, South Carolina, USA, reports that the US Food and Drug Administration (FDA) has provided 510(k) clearance for its maxillofacial surgical guides additively manufactured using its LaserForm® Ti and DuraForm® ProX PA materials.
The new clearance allows for more innovative surgical guide designs that improve performance and is the latest delivery on 3D Systems’ approach to addressing the needs of surgeons performing maxillofacial and reconstructive surgeries.
The company’s surgical guides, produced using these materials as part of its Virtual Surgical Planning VSP® System for craniomaxillofacial (CMF) applications, offers flexibility in design options, and are capable of having lower profile designs to enhance visibility and access in the surgical site, while also possessing improved strength and rigidity. Creating patient-specific surgical guides as part of the VSP System has been shown to save surgeons and patients hours in the operating room [1].
According to 3D Systems, its VSP technology received FDA market clearance as a service-based approach to personalised surgery, combining expertise in medical imaging, surgical simulation, and Additive Manufacturing. The surgeon initiates the process, bringing their clinical knowledge and desired surgical plan to an online web meeting with a 3D Systems biomedical engineer to simulate and plan the surgical procedure. The outcome is a digital plan that is transferred to the operating room via accurate additively manufactured patient-specific models, guides, and templates. 3D Systems has provided VSP solutions or anatomical services in more than 120,000 patient cases.
“Through close collaboration with surgeons and Stryker’s CMF division, we’ve uncovered opportunities to refine VSP guide designs that leverage additional capabilities in our materials portfolio,” stated Menno Ellis, executive vice president, healthcare solutions, 3D Systems.
Ellis added, “Our expert biomedical engineers are now able to design surgical guides tailored to the surgeon’s needs with enhanced properties that can help improve accuracy and facilitate procedures in ways not previously possible. Our powerful VSP System continues to transform surgery – enabling better patient outcomes” [2,3].
Improved surgical guide designs and performance
3D Systems states that its new material options allow for innovative surgical guide designs that improve performance in surgery. It is now possible to design surgical guides with less overall material bulk while improving strength and durability. Mechanical testing shows that titanium cutting and marking guides produced in LaserForm Ti are 20 x stronger than traditional guides [4].
With the improvement in strength, titanium fibula cutting guides can be 70% thinner than traditional guides – facilitating improved access to the surgical site. Similarly, Nylon marking guides produced in DuraForm ProX PA exhibit up to 88% higher toughness [4] – rendering them better able to withstand forces applied during surgery. This enables the creation of thinner guides than previously possible and facilitates a close, snap-like, fit to patient anatomy that cannot be achieved in the traditional material.
Flexibility in treatment options
The new materials are said to allow customisable designs, which are tailored to the cutting and drilling instrumentation to be used in surgery, to assist surgeons with accurately performing cutting and drilling operations. In addition, the guides created using LaserForm Ti and DuraForm ProX PA have been validated with a wider range of cleaning and steam sterilisation options. Users now have more flexibility in using manual or automated cleaning methods, and the devices can be steam sterilised using a wrap or pouch under a wider range of accepted sterilisation cycles. The accepted cleaning and sterilisation techniques validated for the new guide materials offer unprecedented flexibility in assuring safe patient-matched instrumentation is delivered to the operating room.
In 2018, 3D Systems announced an exclusive partnership with Stryker’s CMF division to deliver innovative solutions to the maxillofacial surgeon. The new clearance and enhancements to 3D Systems’ VSP System are the latest delivery on the companies’ shared objectives. The new guide designs and materials are compatible with Facial iD®, Stryker’s market-leading portfolio of patient-matched plating solutions.
References
[1] Sink J, Hamlar D, Kademani D, Khariwala SS: Computer-aided stereolithography for presurgical planning in fibula free tissue reconstruction of the mandible. J Reconstr Microsurg 28:395-404, 2012.
[2] Patel A, Levine J, Brecht L, Saadeh P, Hirsch DL: Digital technologies in mandibular pathology and reconstruction. Atlas Oral Maxillofacial Surg Clin N Am 20:95-106, 2012
[3] Roser SM, Ramachandra S, Blair H, Grist W, Carlson GW, Christensen AM, Weimer KA, Steed MB: The accuracy of virtual surgical planning in free fibula mandibular reconstruction: comparison of planned and final results. J Oral Maxillofac Surg 68:2824-2832, 2010
[4] Proprietary Mechanical Validation Testing Data on file at 3D Systems
















