Tsunami Medical’s early adoption of AM boosts global expansion and innovation
July 28, 2021
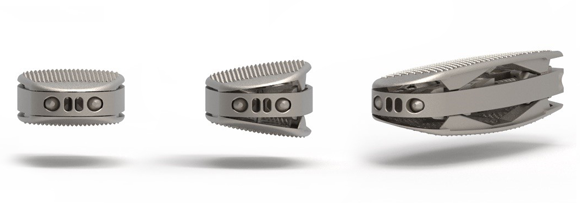
Tsunami Medical, Modena, Italy, a manufacturing company specialising in technological innovation for spinal surgery, has reported continuing growth and global expansion thanks to its early adoption and investment in Additive Manufacturing. As a GE Additive customer, Tsunami Medical has utilised the company’s metal Laser Beam Powder Bed Fusion (PBF-LB) technology to drive its spinal solutions offering.
So far this year, Tsunami Medical has launched nine additively manufactured titanium spinal-fusion implants and a pedicle screw and rod system. The company’s ability to bring so many new products to market is said to be built on the strong working relationship that Stefano Caselli, Tsunami Medical founder and CEO, and his team have established with surgeons over a number of years.
This close collaboration has not only helped the orthopaedic community accept and adopt AM titanium implants, but has become the cornerstone of Tsunami Medical’s R&D strategy.
“We spend a lot of time with surgeons listening to their needs, share creative ideas and experiences and assist in the operating room. It’s well known that the orthopaedic sector has been a champion for metal 3D printing for many years, and over time, trust in Additive Manufacturing has grown,” explained Caselli.
“The deployment of metal Additive Manufacturing continues at pace in orthopaedics. Surgeons and healthcare professionals have become advanced expert users, thanks to their early adoption of the technology. Their comfort level, expertise and desire to innovate result in optimal conditions for accelerating product development and bringing them to market.”
Challenging conventional manufacturing techniques
Tsunami Medical’s second generation of implants incorporates many of the design and manufacturing advantages offered by metal AM, helping to drive further innovation in the orthopaedic sector.
The company’s intervertebral (or interbody) bone ingrowth cages replace the vertebral disc to facilitate a bony fusion of the vertebral segment, and retain or restore balance and stability of the vertebral column. There are several ways to approach the affected vertebral segment, so the company designed cages for each specific surgical approach, designing specific instruments and tools and manufacturing them using AM.
Early interbody cages were made from machined titanium bars owing to the material’s high biocompatibility and following a post-treatment that imparts the appropriate surface roughness, titanium does promote bone ingrowth.
However, titanium cages made by using conventional manufacturing, proved to be too rigid for the application, whereas a certain level of (micro)elasticity of the implant is required to initiate and accelerate bone growth. Additionally, these cages showed serious problems in radiological imaging by showing disturbing scattering. Both the implant stiffness and issues in radiological imaging have been resolved by cages manufactured of polyether ether ketone (PEEK) – a special highly resistant polymer, which became the industry’s standard and preferred choice of surgeons.
PEEK features good biocompatibility, mechanical strength, as well as an elasticity like natural bone, explains Tsunami Medical. Additionally, its radiolucency also allows a good postoperative evaluation. Despite this, PEEK did prove not to be the most suitable material for these applications. Although it addressed the problems of conventionally manufactured titanium cages, it has been proved no bone grows on the PEEK surface. It was, therefore, necessary to reconsider base material and manufacturing processes of interbody cages, which is where additively manufactured titanium cages entered.
By using PBF-LB and Electron Beam Powder Bed Fusion (PBF-EB) it is possible to produce a trabecular surface, which is similar to the natural bone’s structure and optimal for bone ingrowth. Tsunami Medical’s unique net structure design is based on clinical experience and scientific research, which indicate that the ideal pore size for promoting bone ingrowth is between 500 and 700 microns. A size that is said to be only possible by using AM.
This net structure, in combination with the geometrical design of Tsunami Medical’s cage options, offers an elasticity of the implant at least equivalent to PEEK and very close to the micro-elasticity modulus of natural bone. This is the essential requirement for facilitating rapid ingrowth of bone tissue and building the required bone bridge between the vertebral bodies.
Caselli added, “This performance cannot be attained by simply converting an existing design made for PEEK into a 3D-printed one in titanium, which most manufacturers nowadays do; these designs are a lot stiffer than their PEEK alternatives. It is therefore essential to redesign the entire implant to achieve the desired elasticity. A stiff cage can be very risky for the patient, as it can become unseated and penetrate the vertebrae, causing loss of stability of the vertebral segment. Also, subsidence of the implant that causes deterioration of the bone tissue around the implant is an undesirable and high-risk consequence for the patient.”
Driving innovation
Tsunami Medical recently received the regulatory approval (CE Mark) to bring next-generation interbody fusion cages and a screw and rod system to market, following the successfully proven first generation of additively manufactured surgical solutions for spinal fusion.
The second generation of AM solutions includes a line of interbody cages based on successfully proven Bone InGrowth Technology® with options for all surgical approaches. The cages with built-in fixation differentiate from alternatives by using fixation pins instead of screws. Using screws would require substantially more material to fix the screws, whereas by using pins, the elasticity of the implant doesn’t need to be compromised.
The expandable series of interbody cages comes fully assembled from the metal AM machine. They can be extended three-dimensionally, allowing individual adjustment of height and lordosis angle to be achieved with just one instrument.
“With four cages featuring built-in additional fixation features, four expandable ones and the first 3D-printed screw-and-rod system in the world, both for open and MIS procedures, we now are offering a full product portfolio for spinal fusion,” noted Caselli.

Tsunami Medical has also extended its use of AM to pedicle screws, offering one of the world’s first range of fully additively manufactured and CE marked solutions.
The characteristic threading built around a trabecular honeycomb structure is said to be beyond the construction possibilities of conventional manufacturing, while the cannulated and perforated shape, achievable only with AM, yields the necessary strength.
Tsunami’s Ventotene additively manufactured screws exhibit up to 20% higher biocompatibility and mechanical properties when compared to conventionally machined products. Their trabecular structure provides a better bonding on the bone and allow, when necessary, the adding of cement from within the cannulated screw. Since the screws are not made from solid material, they minimise the use of titanium and present a lower radiological footprint, improving the surgeon’s visibility of the surrounding bone tissue. The Ventotene screw system is currently undergoing ASTM testing for the US market.
Scaling global growth
Tsunami Medical is focused on scaling its global growth strategy and is in the process of signing distribution agreements for specific geographical areas. These efforts are being driven by Peter Witke, who joined the company as the company’s Chief Commercial Officer earlier this year, bringing decades of experience in the medical devices sector.
“With our ever-growing portfolio of innovative, Additive Manufacturing-driven, differentiating surgical solutions – which meet all the trends that are currently recognized in the international spine markets – as well as the team’s dedication to stellar customer support, we’re confident in our ability to continue scaling globally over the coming years,” Witke concluded.
















